Ann Rehabil Med.
2015 Jun;39(3):482-487. 10.5535/arm.2015.39.3.482.
MEF2C-Related 5q14.3 Microdeletion Syndrome Detected by Array CGH: A Case Report
- Affiliations
-
- 1Department of Rehabilitation Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- 2Genetics Laboratory, Fertility Center of CHA Gangnam Medical Center, CHA University, Seoul, Korea. shshim@cha.ac.kr
- 3Department of Biomedical Science, College of Life Science, CHA University, Pocheon, Korea.
- KMID: 2165653
- DOI: http://doi.org/10.5535/arm.2015.39.3.482
Abstract
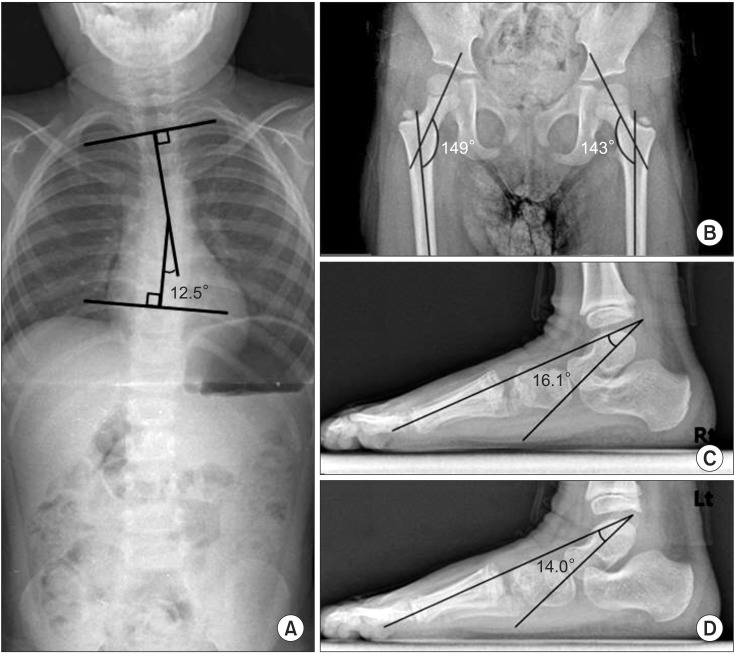
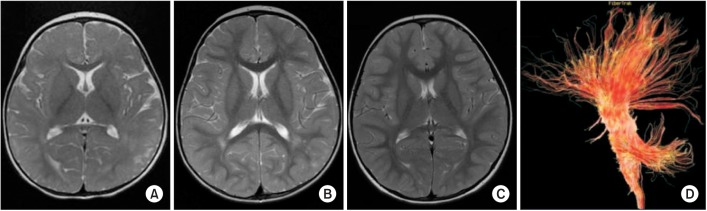
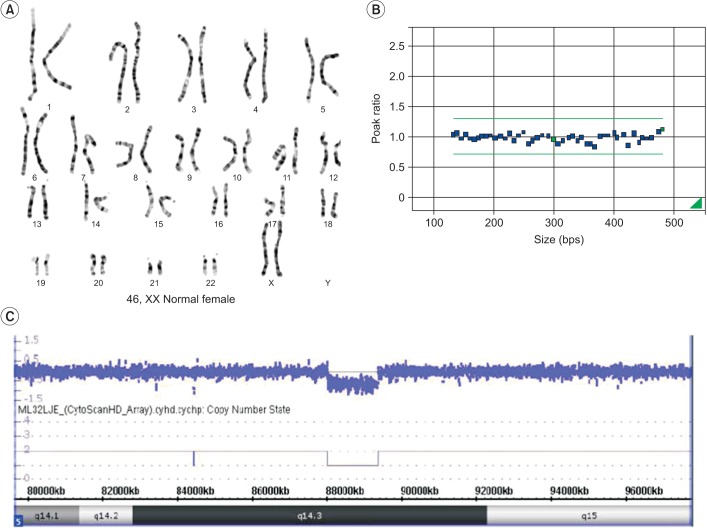
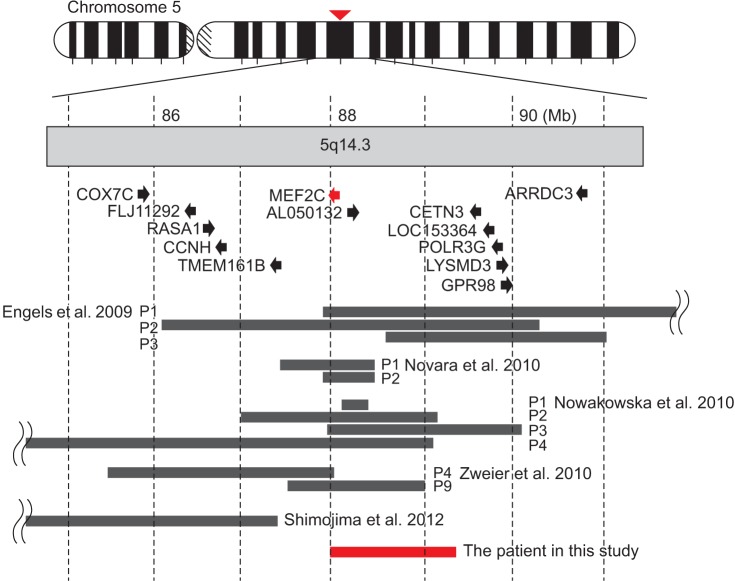
- Genetic screening is being widely applied to trace the origin of global developmental delay or intellectual disability. The 5q14.3 microdeletion has recently been uncovered as a clinical syndrome presenting with severe intellectual disability, limited walking ability, febrile convulsions, absence of speech, and minor brain malformations. MEF2C was suggested as a gene mainly responsible for the 5q14.3 microdeletion syndrome. We present the case of a 6-year-old girl, who is the first patient in Korea with de novo interstitial microdeletions involving 5q14.3, showing the typical clinical features of 5q14.3 microdeletion syndrome with a smaller size of chromosomal involvement compared to the previous reports. The microdeletion was not detected by subtelomeric multiplex ligation-dependent probe amplification, but by array comparative genomic hybridization, which is advisable for the detection of a small-sized genetic abnormality.
MeSH Terms
Figure
Reference
-
1. Hunter AG. Outcome of the routine assessment of patients with mental retardation in a genetics clinic. Am J Med Genet. 2000; 90:60–68. PMID: 10602119.
Article2. Szabo GP, Bessenyei B, Balogh E, Ujfalusi A, Szakszon K, Olah E. Detection of subtelomeric chromosomal rearrangements in idiopathic mental retardation. Orv Hetil. 2010; 151:1091–1098. PMID: 20558358.3. Engels H, Wohlleber E, Zink A, Hoyer J, Ludwig KU, Brockschmidt FF, et al. A novel microdeletion syndrome involving 5q14.3-q15: clinical and molecular cytogenetic characterization of three patients. Eur J Hum Genet. 2009; 17:1592–1599. PMID: 19471318.
Article4. Zweier M, Gregor A, Zweier C, Engels H, Sticht H, Wohlleber E, et al. Mutations in MEF2C from the 5q14.3q15 microdeletion syndrome region are a frequent cause of severe mental retardation and diminish MECP2 and CDKL5 expression. Hum Mutat. 2010; 31:722–733. PMID: 20513142.
Article5. Potthoff MJ, Olson EN. MEF2: a central regulator of diverse developmental programs. Development. 2007; 134:4131–4140. PMID: 17959722.
Article6. Shimojima K, Okumura A, Mori H, Abe S, Ikeno M, Shimizu T, et al. De novo microdeletion of 5q14.3 excluding MEF2C in a patient with infantile spasms, microcephaly, and agenesis of the corpus callosum. Am J Med Genet A. 2012; 158A:2272–2276. PMID: 22848023.7. Nowakowska BA, Obersztyn E, Szymanska K, Bekiesinska-Figatowska M, Xia Z, Ricks CB, et al. Severe mental retardation, seizures, and hypotonia due to deletions of MEF2C. Am J Med Genet B Neuropsychiatr Genet. 2010; 153B:1042–1051. PMID: 20333642.8. Novara F, Beri S, Giorda R, Ortibus E, Nageshappa S, Darra F, et al. Refining the phenotype associated with MEF2C haploinsufficiency. Clin Genet. 2010; 78:471–477. PMID: 20412115.9. Saitsu H, Igarashi N, Kato M, Okada I, Kosho T, Shimokawa O, et al. De novo 5q14.3 translocation 121.5-kb upstream of MEF2C in a patient with severe intellectual disability and early-onset epileptic encephalopathy. Am J Med Genet A. 2011; 155A:2879–2884. PMID: 21990267.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Overgrowth Syndrome with 9q22.3 Microdeletion Detected by Microarray Comparative Genomic Hybridization
- Identification of a Novel Deletion Region in 3q29 Microdeletion Syndrome by Oligonucleotide Array Comparative Genomic Hybridization
- Using Array-Based Comparative Genomic Hybridization to Diagnose Pallister-Killian Syndrome
- Array-based Comparative Genomic Hybridization and Its Application to Cancer Genomes and Human Genetics
- Analysis of Chromosomal Aberrations in Lung Cancer Cell Line, NCI-H1373