J Vet Sci.
2015 Jun;16(2):135-143. 10.4142/jvs.2015.16.2.135.
Anti-melanogenic effects of black, green, and white tea extracts on immortalized melanocytes
- Affiliations
-
- 1Department of Public Health, Keimyung University, Daegu 704-701, Korea. yckim@kmu.ac.kr
- 2Department of Medical Skin Care, Daegu Mirae College, Gyeongsan 712-716, Korea.
- KMID: 2164521
- DOI: http://doi.org/10.4142/jvs.2015.16.2.135
Abstract
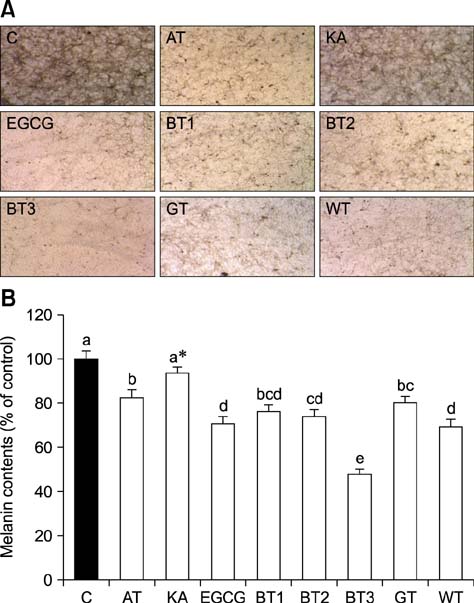
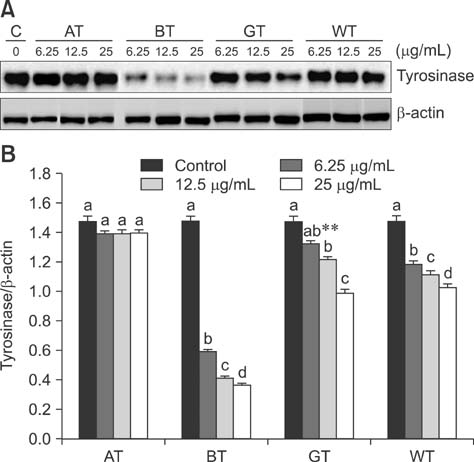
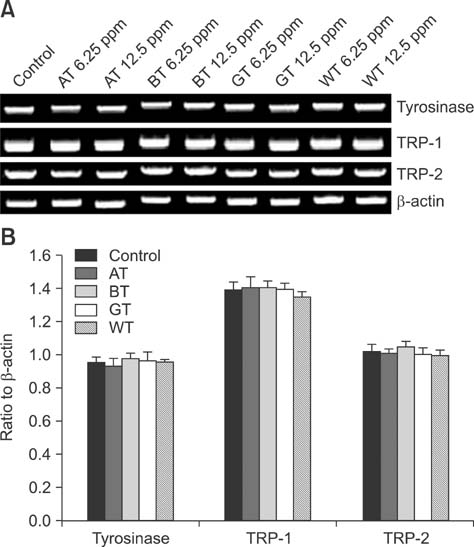
- Tea contains polyphenols and is one of the most popular beverages consumed worldwide. Because most tyrosinase inhibitors that regulate melanogenesis are phenol/catechol derivatives, this study investigated the inhibitory effects of Camellia sinensis water extracts (CSWEs), including black tea, green tea, and white tea extracts, on melanogenesis using immortalized melanocytes. CSWEs inhibited melanin accumulation and melanin synthesis along with tyrosinase activity in a concentration-dependent manner. These inhibitory effects were superior to those of arbutin, a well-known depigmenting agent. The anti-melanogenic activity of black (fermented) tea was higher than that of a predominant tea catecholamine, epigallocatechin gallate. CSWEs, especially black tea extract, decreased tyrosinase protein levels in a concentration-dependent manner. These results suggest that the anti-melanogenic effect of CSWEs is mediated by a decrease in both tyrosinase activity and protein expression, and may be augmented by fermentation. Thus, CSWEs could be useful skin-whitening agents in the cosmetic industry.
Keyword
MeSH Terms
Figure
Reference
-
1. Al-Azzawie HF, Alhamdani MSS. Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sci. 2006; 78:1371–1377.
Article2. Alcázar A, Ballesteros O, Jurado JM, Pablos F, Martín MJ, Vilches JL, Navalón A. Differentiation of green, white, black, oolong, and pu-erh teas according to their free amino acids content. J Agric Food Chem. 2007; 55:5960–5965.
Article3. Ando H, Kondoh H, Ichihashi M, Hearing VJ. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J Invest Dermatol. 2007; 127:751–761.
Article4. Aoki Y, Tanigawa T, Abe H, Fujiwara Y. Melanogenesis inhibition by an oolong tea extract in B16 mouse melanoma cells and UV-induced skin pigmentation in brownish guinea pigs. Biosci Biotechnol Biochem. 2007; 71:1879–1885.
Article5. Bennett DC, Cooper PJ, Hart IR. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int J Cancer. 1987; 39:414–418.
Article6. Chakraborty AK, Funasaka Y, Komoto M, Ichihashi M. Effect of arbutin on melanogenic proteins in human melanocytes. Pigment Cell Res. 1998; 11:206–212.
Article7. Chang TS, Lin JJ. Inhibitory effect of danazol on melanogenesis in mouse B16 melanoma cells. Arch Pharm Res. 2010; 33:1959–1965.
Article8. Cullen MK. Genetic epidermal syndromes: disorders characterized by lentigines. In : Nordlund JJ, Boissy RE, Hearing VJ, King RA, Ortonne JP, editors. The Pigmentary System: Physiology and Pathophysiology. New York: Oxford University Press;1998. p. 760–766.9. Davies R, Massey RC, McWeeny DJ. The catalysis of the N-nitrosation of secondary amines by nitrosophenols. Food Chem. 1980; 6:115–122.
Article10. Draelos ZD. Skin lightening preparations and the hydroquinone controversy. Dermatol Ther. 2007; 20:308–313.
Article11. Folin O, Denis W. On phosphotungstic-phosphomolybdic compounds as color reagents. J Biol Chem. 1912; 12:239–243.
Article12. Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992; 21:334–350.
Article13. Hearing VJ. Biogenesis of pigment granules: a sensitive way to regulate melanocyte function. J Dermatol Sci. 2005; 37:3–14.
Article14. Kasamatsu S, Hachiya A, Nakamura S, Yasuda Y, Fujimori T, Takano K, Moriwaki S, Hase T, Suzuki T, Matsunaga K. Depigmentation caused by application of the active brightening material, rhododendrol, is related to tyrosinase activity at a certain threshold. J Dermatol Sci. 2014; 76:16–24.
Article15. Kim DS, Park SH, Kwon SB, Li K, Youn SW, Park KC. (-)-Epigallocatechin-3-gallate and hinokitiol reduce melanin synthesis via decreased MITF production. Arch Pharm Res. 2004; 27:334–339.
Article16. Kim YJ, Uyama H. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci. 2005; 62:1707–1723.
Article17. Lee YS, Kim HK, Lee KJ, Jeon HW, Cui S, Lee YM, Moon BJ, Kim YH, Lee YS. Inhibitory effect of glyceollin isolated from soybean against melanogenesis in B16 melanoma cells. BMB Rep. 2010; 43:461–467.
Article18. Lim JT. Treatment of melasma using kojic acid in a gel containing hydroquinone and glycolic acid. Dermatol Surg. 1999; 25:282–284.
Article19. Maeda K, Fukuda M. Arbutin: mechanism of its depigmenting action in human melanocyte culture. J Pharmacol Exp Ther. 1996; 276:765–769.20. Mallick S, Singh SK, Sarkar C, Saha B, Bhadra R. Human placental lipid induces melanogenesis by increasing the expression of tyrosinase and its related proteins in vitro. Pigment Cell Res. 2005; 18:25–33.
Article21. Menter JM, Etemadi AA, Chapman W, Hollins TD, Willis I. In vivo depigmentation by hydroxybenzene derivatives. Melanoma Res. 1993; 3:443–449.22. Ministry of Food and Drug Safety (KR). Permission levels and experimental methods of heavy metals for herb medicines, etc. Notice 2008-2. 2008. 01. 08.23. No JK, Soung DY, Kim YJ, Shim KH, Jun YS, Rhee SH, Yokozawa T, Chung HY. Inhibition of tyrosinase by green tea components. Life Sci. 1999; 65:PL241–PL246.
Article24. Ortonne JP, Nordlund JJ. Mechanisms that cause abnormal skin color. In : Nordlund JJ, Boissy RE, Hearing VJ, King RA, Ortonne JP, editors. The Pigmentary System: Physiology and Pathophysiology. New York: Oxford University Press;1998. p. 489–502.25. Passi S, Nazzaro-Porro M. Molecular basis of substrate and inhibitory specificity of tyrosinase: phenolic compounds. Br J Dermatol. 1981; 104:659–665.
Article26. Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995; 22:375–383.
Article27. Sasaki M, Kondo M, Sato K, Umeda M, Kawabata K, Takahashi Y, Suzuki T, Matsunaga K, Inoue S. Rhododendrol, a depigmentation-inducing phenolic compound, exerts melanocyte cytotoxicity via a tyrosinase-dependent mechanism. Pigment Cell Melanoma Res. 2014; 27:754–763.
Article28. Serafini M, Ghiselli A, Ferro-Luzzi A. In vivo antioxidant effect of green and black tea in man. Eur J Clin Nutr. 1996; 50:28–32.29. Seyoum A, Asres K, El-Fiky FK. Structure-radical scavenging activity relationships of flavonoids. Phytochemistry. 2006; 67:2058–2070.
Article30. Sheffield MV, Yee H, Dorvault CC, Weilbaecher KN, Eltoum IA, Siegal GP, Fisher DE, Chhieng DC. Comparison of five antibodies as markers in the diagnosis of melanoma in cytologic preparations. Am J Clin Pathol. 2002; 118:930–936.
Article31. Solano F, Briganti S, Picardo M, Ghanem G. Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006; 19:550–571.
Article32. Sun CL, Yuan JM, Koh WP, Yu MC. Green tea, black tea and breast cancer risk: a meta-analysis of epidemiological studies. Carcinogenesis. 2006; 27:1310–1315.
Article33. Thring TS, Hili P, Naughton DP. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement Altern Med. 2009; 9:27.
Article34. Uehara S, Mizutani Y, Nakade M, Sakata O, Sasaki I, Arakane K, Suzuki T, Uchida R, Tokutake S. Inhibitory effects of proanthocyanidin-rich extracts from grape seeds on melanogenesis. J Jpn Cosmet Sci Soc. 2003; 27:247–255.35. Urabe K, Nakayama J, Hori Y. Mixed epidermal and dermal hypermelanoses. In : Nordlund JJ, Boissy RE, Hearing VJ, King RA, Ortonne JP, editors. The Pigmentary System: Physiology and Pathophysiology. New York: Oxford University Press;1998. p. 909–911.36. Yamaoka Y, Ohguchi K, Itoh T, Nozawa Y, Akao Y. Effects of theaflavins on melanin biosynthesis in mouse B16 melanoma cells. Biosci Biotechnol Biochem. 2009; 73:1429–1431.
Article37. Zhong S, Wu Y, Ahn SM, Zhao J, Wang K, Yang S, Yeon JH, Zhu X. Depigmentation of melanocytes by the treatment of extracts from traditional Chinese herbs: a cell culture assay. Biol Pharm Bull. 2006; 29:1947–1951.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transactivation of peroxisome proliferator-activated receptor alpha by green tea extracts
- Effects of Green tea (-)-epigallocatechin-3-gallate and Polyphenol on the Proliferation and Apoptosis of Cultured Human Keratinocytes, Melanocytes, Fibroblasts, Endothelial Cells, and Human Epidermoid Carcinoma Cells
- Evaluation of the Anti-inflammatory Effect of a Moisturizer Containing Green-Tea Extracts
- Human Skin Safety Test of Green Tea Cell Extracts in Condition of Allergic Contact Dermatitis
- The Effects of Extracts from Green Tea, Guajava Leaves and Rose Petals on Allergic Rhinitis: A Randomized Double Blind Study