J Vet Sci.
2015 Mar;16(1):113-120. 10.4142/jvs.2015.16.1.113.
The production and distribution of IL-6 and TNF-alpha in subcutaneous adipose tissue and their correlation with serum concentrations in Welsh ponies with equine metabolic syndrome
- Affiliations
-
- 1Electron Microscope Laboratory, Wroclaw University of Environmental and Life Sciences, Wroclaw 51-631, Poland. basinskakatarzyna@wp.pl
- 2Department and Clinic of Veterinary Surgery, Wroclaw University of Environmental and Life Sciences, Wroclaw 50-366, Poland.
- KMID: 2160828
- DOI: http://doi.org/10.4142/jvs.2015.16.1.113
Abstract
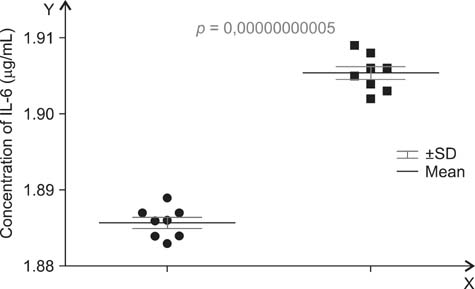
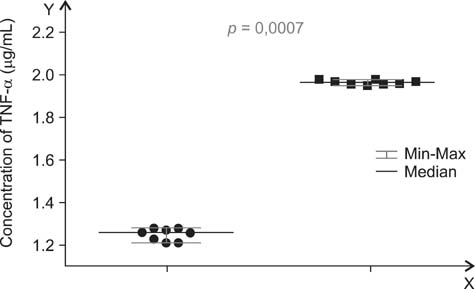
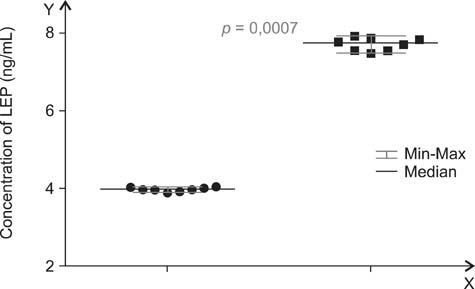
- A main symptom of equine metabolic syndrome (EMS) in ponies is pathological obesity characterized by abnormal accumulation of fat deposits and inflammation. In this study, we analyzed the expression of two pro-inflammatory cytokines, interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-alpha), in subcutaneous adipose tissue and the correlation with serum concentrations in peripheral blood of Welsh ponies. Based on clinical examination findings, the animals were divided into two groups: ponies affected with EMS (n = 8) and obese ponies (n = 8). The adipose tissue was examined using immunohistochemical analysis while concentrations IL-6 and TNF-alpha were measured using enzyme-linked immunosorbent assays (ELISAs). Additionally, histological characterization of the adipose tissue was performed. The results obtained showed that IL-6 expression in adipose tissue biopsies derived from animals with EMS was enhanced while TNF-alpha levels of both groups were comparable. Compared to the obese ponies, EMS animals also had significantly elevated levels of serum IL-6 and TNF-alpha. Histological analysis revealed macrophage infiltration and fibrosis in adipose tissue preparations from the EMS group. These data suggest that IL-6 may play a key role in the course of EMS in Welsh ponies. Our findings also demonstrated that analysis of pro-inflammatory cytokines levels in serum may serve as an additional tool for diagnosing EMS.
Keyword
MeSH Terms
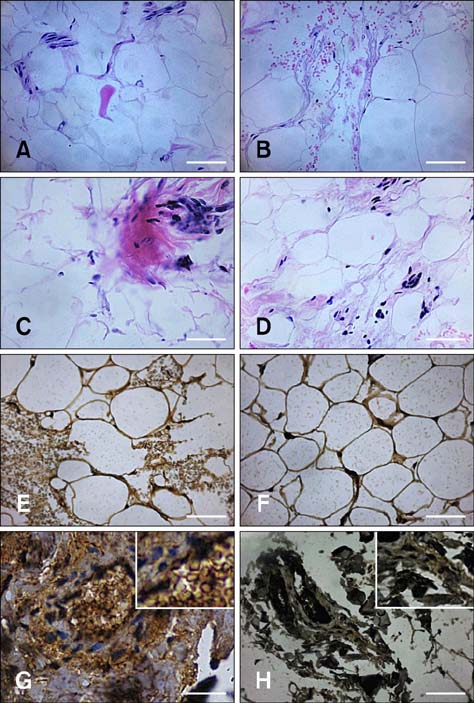
Figure
Reference
-
1. Agrawal S, Gollapudi S, Su H, Gupta S. Leptin activates human B cells to secrete TNF-α, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J Clin Immunol. 2011; 31:472–478.
Article2. Barnes TC, Anderson ME, Moots RJ. The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int J Rheumatol. 2011; 2011:721608.
Article3. Bertin E, Nguyen P, Guenounou M, Durlach V, Potron G, Leutenegger M. Plasma levels of tumor necrosis factor-alpha (TNF-α) are essentially dependent on visceral fat amount in type 2 diabetic patients. Diabetes Metab J. 2000; 26:178–182.4. Carter RA, Geor RJ, Staniar WB, Cubitt TA, Harris PA. Apparent adiposity assessed by standardised scoring systems and morphometric measurements in horses and ponies. Vet J. 2009; 179:204–210.
Article5. Dugdale AH, Curtis GC, Cripps P, Harris PA, Argo CM. Effect of dietary restriction on body condition, composition and welfare of overweight and obese pony mares. Equine Vet J. 2010; 42:600–610.
Article6. Frank N. Equine metabolic syndrome. Vet Clin North Am Equine Pract. 2011; 27:73–92.
Article7. Frank N, Geor RJ, Bailey SR, Durham AE, Johnson PJ. Equine metabolic syndrome. J Vet Intern Med. 2010; 24:467–475.
Article8. Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006; 8:Suppl 2. S3.9. Gordon ME, McKeever KH, Betros CL, Manso Filho HC. Plasma leptin, ghrelin and adiponectin concentrations in young fit racehorses versus mature unfit standardbreds. Vet J. 2007; 173:91–100.
Article10. Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006; 83:Suppl. 461S–465S.
Article11. Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes and vascular diseases. Eur Heart J. 2008; 29:2959–2971.
Article12. Henneke DR, Potter GD, Kreider JL, Yeates BF. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet J. 1983; 15:371–372.
Article13. Hotamisligil GS, Spiegelman BM. Tumor necrosis factor α: a key component of the obesity-diabetes link. Diabetes. 1994; 43:1271–1278.
Article14. Johnson PJ. The equine metabolic syndrome peripheral Cushing's syndrome. Vet Clin North Am Equine Pract. 2002; 18:271–293.15. Johnson PJ, Wiedmeyer CE, Messer NT, Ganjam VK. Medical implications of obesity in horses-lessons for human obesity. J Diabetes Sci Technol. 2009; 3:163–174.
Article16. Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006; 8:Suppl 2. S2.17. Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001; 280:E745–E751.
Article18. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004; 89:2548–2556.
Article19. Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM. Leptin regulates proinflammatory immune responses. FASEB J. 1998; 12:57–65.
Article20. Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverse starvation-induced immunosupresion. Nature. 1998; 394:897–901.
Article21. Marycz K, Moll E, Grzesiak J. Influence of functional nutrients on insulin resistance in horses with equine metabolic syndrome. Pak Vet J. 2014; 34:189–192.22. Marycz K, Toker NY, Czogaa J, Michalak I, Nicpon J, Grzesiak J. An investigation of the elemental composition of horse hair affected by equine metabolic syndrome (EMS) using SEM EDX and ICP-OES. J Anim Vet Adv. 2013; 12:146–152.23. Nieto-Vazquez I, Fernández-Veledo S, Krämer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem. 2008; 114:183–194.
Article24. Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003; 14:561–566.
Article25. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines inflammation and metabolic disease. Nat Rev Immunol. 2011; 11:85–97.26. Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007; 48:751–762.
Article27. Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008; 14:741–751.
Article28. Suagee JK, Corl BA, Crisman MV, Hulver MW, McCutcheon LJ, Geor RJ. Effects of acute hyperinsulinemia on inflammatory proteins in horses. Vet Immunol Immunopathol. 2011; 142:141–146.
Article29. Suagee JK, Corl BA, Geor RJ. A potential role for pro-inflammatory cytokines in the development of insulin resistance in horses. Animals (Basel). 2012; 2:243–260.
Article30. Treiber K, Carter R, Gay L, Williams C, Geor R. Inflammatory and redox status of ponies with a history of pasture-associated laminitis. Vet Immunol Immunopathol. 2009; 129:216–220.
Article31. Wiesberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112:1796–1808.
Article32. Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004; 15:2792–2800.
Article33. Wyse CA, McNie KA, Tannahill VJ, Murray JK, Love S. Prevalence of obesity in riding horses in Scotland. Vet Rec. 2008; 162:590–591.
Article34. Yang R, Barouch LA. Leptin signaling and obesity: cardiovascular consequences. Circ Res. 2007; 101:545–559.35. Vick MM, Adams AA, Murphy BA, Sessions DR, Horohov DW, Cook RF, Shelton BJ, Fitzgerald BP. Relationships among inflammatory cytokines, obesity, and insulin sensitivity in the horse. J Anim Sci. 2007; 85:1144–1155.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Upregulation of Macrophage Migration Inhibitory Factor (MIF) Production from Peripheral Blood Mononuclear Cells (PBMCs) Stimulated by Tumor Necrosis Factor (TNF)-alpha in Patients with Behcet's Syndrome
- Comparison of Cytokine Expressions among Kawasaki Disease and Its Symptom-related Diseases
- Serum adiponectin concentration according to visceral fat amount and its relationship of metabolic risk factors in premenopausal obese women
- Clinical Study of the Correlation of Tumor Necrosis Factor alpha and the Proteinuria of Henoch-Schonlein Nephritis and Idiopathic Nephrotic Syndrome
- Sex-dependent Depot Differences in Adipose Tissue Development and Function; Role of Sex Steroids