J Vet Sci.
2015 Mar;16(1):47-55. 10.4142/jvs.2015.16.1.47.
Autologous processed plasma: cytokine profile and effects upon injection into healthy equine joints
- Affiliations
-
- 1Department of Internal Medicine, School of Veterinary Medicine and Animal Science, University of Sao Paulo, Sao Paulo 05508 270, Brazil. baccarin@usp.br
- 2Department of Biochemistry, Federal University of Sao Paulo, Sao Paulo 04021 001, Brazil.
- 3Department of Pathology, School of Veterinary Medicine and Animal Science, University of Sao Paulo, Sao Paulo 05508 270, Brazil.
- KMID: 2160820
- DOI: http://doi.org/10.4142/jvs.2015.16.1.47
Abstract
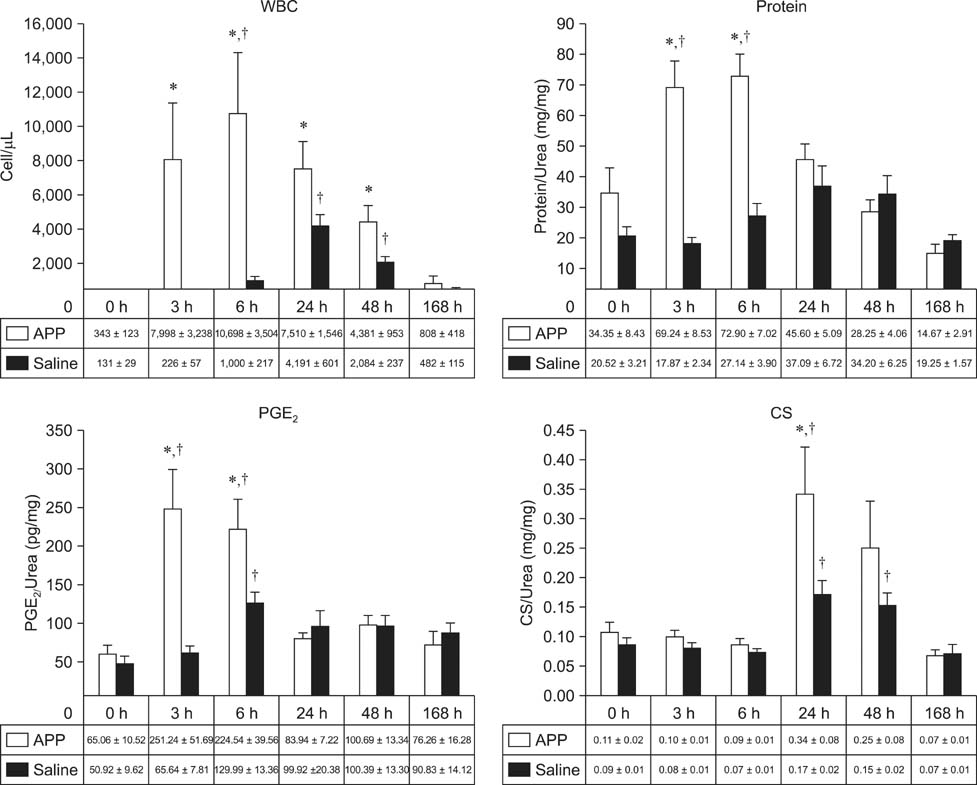
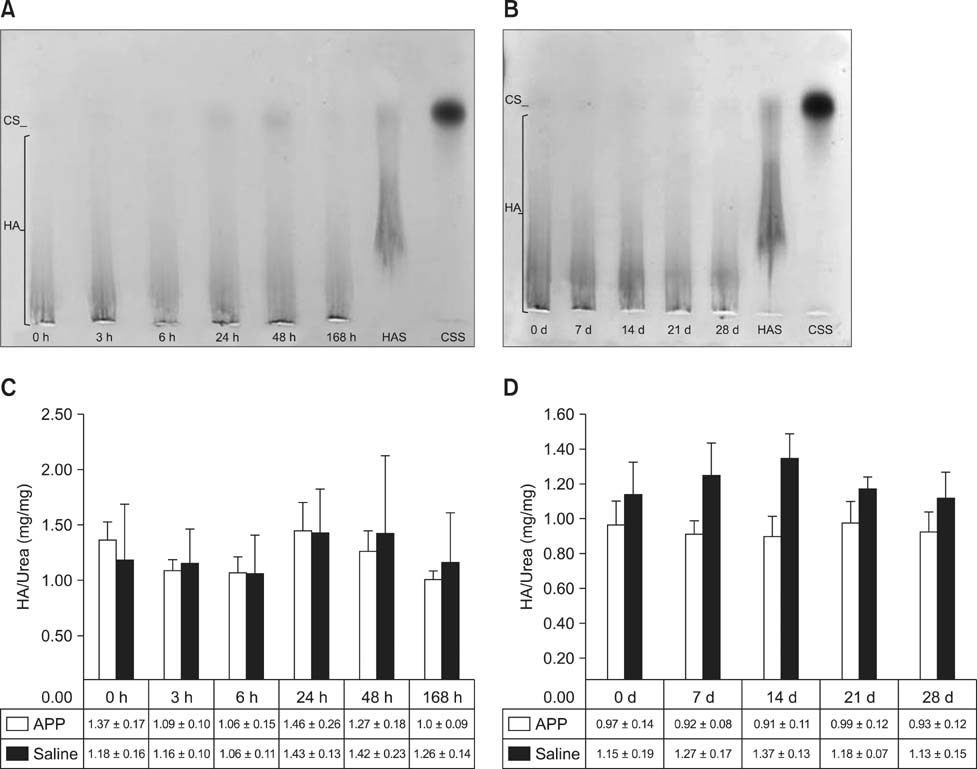
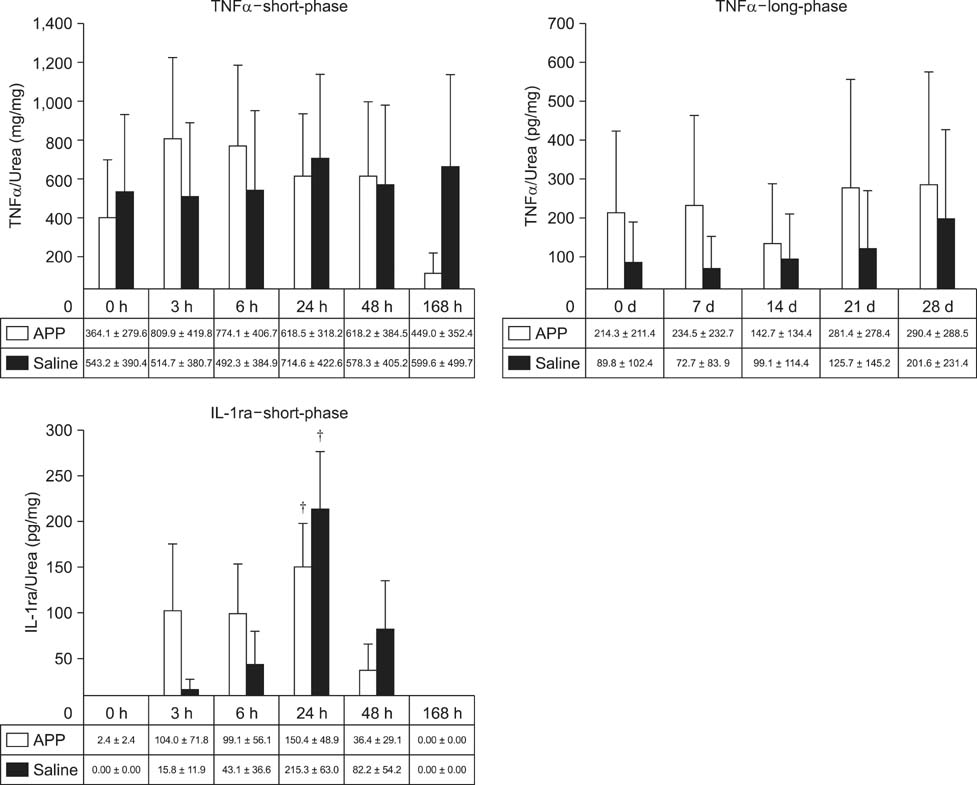
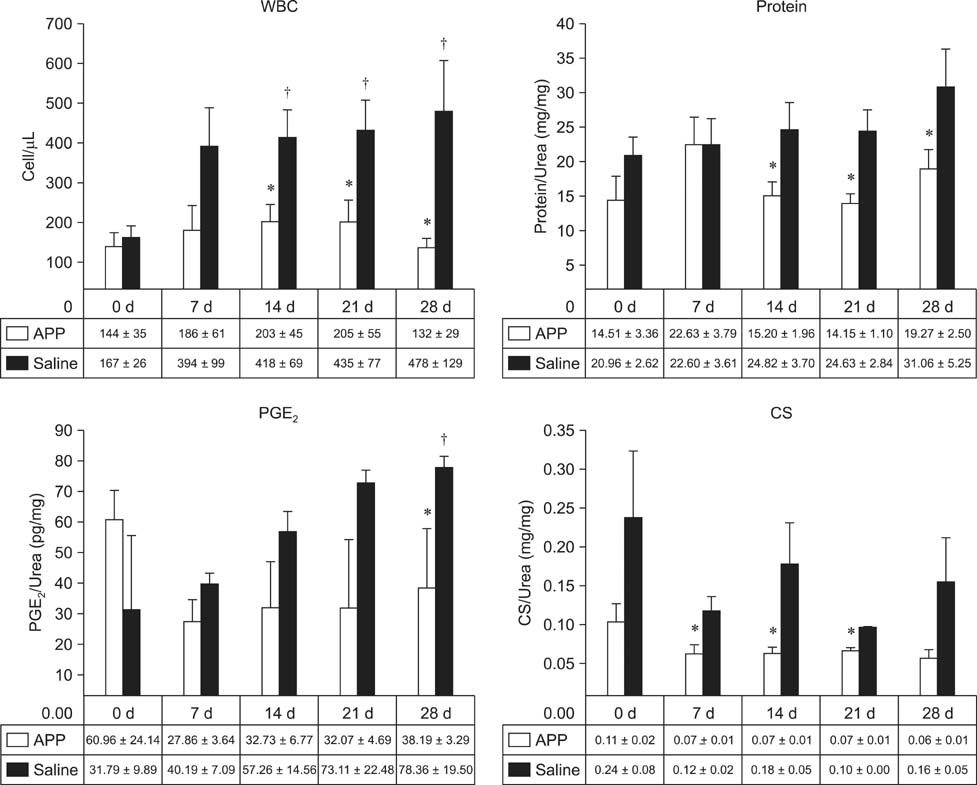
- This experimental controlled study was performed to evaluate the composition of autologous processed plasma (APP), and the effects of APP intra-articular injection into healthy equine metacarpophalangeal joints. The effects on joints were analysed with a short-phase protocol and a prolonged-phase protocol using saline-injected joints as controls. For the short protocol, horses received one intra-articular APP injection. Synovial fluid samples were collected prior to the injection and 3, 6, 24, 48, and 16 h after treatment. For the prolonged protocol, the joints received three weekly injections of APP, and samples were collected at 0, 7, 14, 21, and 28 days before APP administration. IL1-ra level was found to be increased in APP compared to plasma. Upon intra-articular administration of APP, transient (up to 24 h) increases in white blood cell (WBC) counts along with elevated protein and prostaglandin E2 (PGE2) concentrations were observed in the treated joints. Over the 28-day observation period, APP did not elicit changes relative to baseline levels, but WBC counts, PGE2 and chondroitin sulphate concentrations were lower than those found in the control. In conclusion, APP intra-articular injection induced a mild and transitory inflammatory response but no inflammation reaction was observed over a longer period of treatment and observation.
MeSH Terms
Figure
Cited by 1 articles
-
Hyaluronic acid has chondroprotective and joint-preserving effects on LPS-induced synovitis in horses
Henrique M. Neuenschwander, Juliana J. Moreira, Cynthia P. Vendruscolo, Joice Fülber, Sarah R. T. Seidel, Yara M. Michelacci, Raquel Y. A. Baccarin
J Vet Sci. 2019;20(6):. doi: 10.4142/jvs.2019.20.e67.
Reference
-
1. Arend WP. Interleukin-1 receptor antagonist: discovery, structure and properties. Prog Growth Factor Res. 1990; 2:193–205.
Article2. Baccarin RYA, Rasera L, Machado TSL, Michelacci YM. Relevance of synovial fluid chondroitin sulphate as a biomarker to monitor polo pony joints. Can J Vet Res. 2014; 78:50–60.3. Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005; 64:1263–1267.
Article4. Berenbaum F. Targeted therapies in osteoarthritis: a systematic review of the trials on www.clinicaltrials.gov. Best Pract Res Clin Rheumatol. 2010; 24:107–119.
Article5. Billinghurst RC, Fretz PB, Gordon JR. Induction of intra-articular tumour necrosis factor during acute inflammatory responses in equine arthritis. Equine Vet J. 1995; 27:208–216.
Article6. Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophageproduced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006; 8:R187.7. Brossi PM, Baccarin RYA, Massoco CO. Do blood components affect the production of reactive oxygen species (ROS) by equine synovial cells in vitro? Pesqui Vet Bras. 2012; 32:1355–1360.
Article8. Carmalt JL, Bell CD, Tatarniuk DM, Suri SS, Singh B, Waldner C. Comparison of the response to experimentally induced short-term inflammation in the temporomandibular and metacarpophalangeal joints of horses. Am J Vet Res. 2011; 72:1586–1591.
Article9. Caron JP. Osteoarthritis. In : Ross MW, Dyson SJ, editors. Diagnosis and management of Lameness in the Horse. 1st ed. Philadelphia: Saunders;2003. p. 572–594.10. Carter DB, Deibel MR Jr, Dunn CJ, Tomich CSC, Laborde AL, Slightom JL, Berger AE, Bienkowski MJ, Sun FF, McEwan RN, Harris PKW, Yem AW, Waszak GA, Chosay JG, Sieu LC, Hardee MM, Zurcher-Neely HA, Reardon IM, Heinrikson RL, Truesdell SE, Shelly JA, Eessalu TE, Taylor BM, Tracey DE. Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature. 1990; 344:633–638.
Article11. de Grauw JC, van de Lest CH, van Weeren PR. Inflammatory mediators and cartilage biomarkers in synovial fluid after a single inflammatory insult: a longitudinal experimental study. Arthritis Res Ther. 2009; 11:R35.
Article12. Frisbie DD, Al-Sobayil F, Billinghurst RC, Kawcak CE, McIlwraith CW. Changes in synovial fluid and serum biomarkers with exercise and early osteoarthritis in horses. Osteoarthritis Cartilage. 2008; 16:1196–1204.
Article13. Frisbie DD, Kawcak CE, Werpy NM, Park RD, McIlwraith CW. Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am J Vet Res. 2007; 68:290–296.
Article14. Garlanda C, Riva F, Bonavita E, Mantovani A. Negative regulatory receptors of the IL-1 family. Semin Immunol. 2013; 25:408–415.
Article15. Gough MR, Munroe GA, Mayhew IG. Urea as measure of dilution of equine synovial fluid. Equine Vet J. 2002; 34:76–79.16. Hawkins DL, Mackay RJ, Gum GG, Colahan PT, Meyer J. Effects of intra-articularly administered endotoxin on clinical signs of disease and synovial fluid tumor necrosis factor, interleukin 6, and prostaglandin E2 values in horses. Am J Vet Res. 1993; 54:379–386.17. Hraha TH, Doremus KM, McIlwraith CW, Frisbie DD. Autologous conditioned serum: the comparative cytokine profiles of two commercial methods (IRAP and IRAP II) using equine blood. Equine Vet J. 2011; 43:516–521.
Article18. Kamm JL, Nixon AJ, Witte TH. Cytokine and catabolic enzyme expression in synovium, synovial fluid and articular cartilage of naturally osteoarthritic equine carpi. Equine Vet J. 2010; 42:693–699.
Article19. Kraus VB, Stabler TV, Kong SY, Varju G, McDaniel G. Measurement of synovial fluid volume using urea. Osteoarthritis Cartilage. 2007; 15:1217–1220.
Article20. Lathrop JT, Anderson NL, Anderson NG, Hammond DJ. Therapeutic potential of the plasma proteome. Curr Opin Mol Ther. 2010; 5:250–257.21. Ley C, Ekman S, Elmén A, Nilsson G, Eloranta ML. Interleukin-6 and tumor necrosis factor in synovial fluid from horses with carpal joint pathology. J Vet Med A Physiol Pathol Clin Med. 2007; 54:346–351.
Article22. Liberg P, Magnusson LE, Schougaard H. Studies on the synovia in healthy horses with particular reference to the protein composition. Equine Vet J. 1977; 9:87–91.
Article23. Machado TSL, Correia da Silva LCL, Baccarin RYA, Michelacci YM. Synovial fluid chondroitin sulphate indicates abnormal joint metabolism in asymptomatic osteochondritic horses. Equine Vet J. 2012; 44:404–411.
Article24. Martel-Pelletier J, Alaaeddine N, Pelletier JP. Cytokines and their role in the pathophysiology of osteoarthritis. Front Biosci. 1999; 4:D694–D703.
Article25. Meijer H, Reinecke J, Becker C, Tholen G, Wehling P. The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm Res. 2003; 52:404–407.
Article26. Misheff MM, Stover SM. A comparison of two techniques for arthrocentesis of the equine metacarpophalangeal joint. Equine Vet J. 1991; 23:273–276.
Article27. Morris EA, McDonald BS, Webb AC, Rosenwasser LJ. Identification of interleukin-1 in equine osteoarthritic joint effusions. Am J Vet Res. 1990; 51:59–64.28. Palmer JL, Bertone AL, McClain H. Assessment of glycosaminoglycan concentration in equine synovial fluid as a marker of joint disease. Can J Vet Res. 1995; 59:205–212.29. Pascual C, Bredle D, Karzai W, Meier-Hellmann A, Oberhoffer M, Reinhart K. Effect of plasma and LPS on respiratory burst of neutrophils in septic patients. Intensive Care Med. 1988; 24:1181–1186.
Article30. Rasera L, Massoco CO, Landgraf RG, Baccarin RYA. Exercise induced apoptosis and necrosis in the synovial fluid cells of athletic horses. Pesqui Vet Bras. 2008; 28:231–236.31. Riggs CM. Osteochondral injury and joint disease in the athletic horse. Equine Vet Educ. 2006; 18:100–112.
Article32. Ross TN, Kisiday JD, Hess T, McIlwraith CW. Evaluation of the inflammatory response in experimentally induced synovitis in the horse: a comparison of recombinant equine interleukin 1 beta and lipopolysaccharide. Osteoarthritis Cartilage. 2012; 20:1583–1590.
Article33. Rutgers M, Saris DBF, Dhert WJA, Creemers LB. Cytokine profile of autologous conditioned serum for treatment of osteoarthritis, in vitro effects on cartilage metabolism and intra-articular levels after injection. Arthritis Res Ther. 2010; 12:R114.
Article34. Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010; 6:625–635.
Article35. Textor J. Autologous biologic treatment for equine musculoskeletal injuries: platelet-rich plasma and IL-1 receptor antagonist protein. Vet Clin North Am Equine Pract. 2011; 27:275–298.
Article36. Textor JA, Tablin F. Intra-articular use of a platelet-rich product in normal horses: clinical signs and cytologic responses. Vet Surg. 2013; 42:499–510.
Article37. Tulamo RM, Bramlage LR, Gabel AA. Sequential clinical and synovial fluid changes associated with acute infectious arthritis in the horse. Equine Vet J. 1989; 21:325–331.
Article38. Tulamo RM, Heiskanen T, Salonen M. Concentration and molecular weight distribution of hyaluronate in synovial fluid from clinically normal horses and horses with disease joints. Am J Vet Res. 1994; 55:710–715.39. van den Boom R, Brama PAJ, Kiers GH, DeGroot J, Barneveld A, van Weeren PR. The influence of repeated arthrocentesis and exercise on matrix metalloproteinase and tumour necrosis factor α activities in normal equine joints. Equine Vet J. 2004; 36:155–159.40. van den Boom R, van de Lest CHA, Bull S, Brama PAJ, van Weeren PR, Barneveld A. Influence of repeated arthrocentesis and exercise on synovial fluid concentrations of nitric oxide, prostaglandin E2 and glycosaminoglycans in healthy equine joints. Equine Vet J. 2005; 37:250–256.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Low Dose Estrogen Replacement Therapy on the Lipid Profile of Postmenopausal Women
- Characterization of the cytokine profile of platelet rich plasma (PRP) and PRP-induced cell proliferation and migration: Upregulation of matrix metalloproteinase-1 and -9 in HaCaT cells
- Autologous Platelet-Poor Plasma Gel for Injection Laryngoplasty
- Differential Role of Transforming Growth Factor-beta in an Osteoarthritic or a Healthy Joint
- Treatment of Recalcitrant Medial and Lateral Epicondylitis with Autologous Platelet Rich Plasma; Preliminary Report