J Korean Med Sci.
2015 Jun;30(6):770-778. 10.3346/jkms.2015.30.6.770.
The Proteomic Analysis of Human Placenta with Pre-eclampsia and Normal Pregnancy
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Ajou University Medical School, Suwon, Korea. yangji@ajou.ac.kr
- 2Department of Obstetrics and Gynecology, Kangdong Sacred Heart Hospital, Hallym University Medical School, Seoul, Korea.
- KMID: 2160607
- DOI: http://doi.org/10.3346/jkms.2015.30.6.770
Abstract
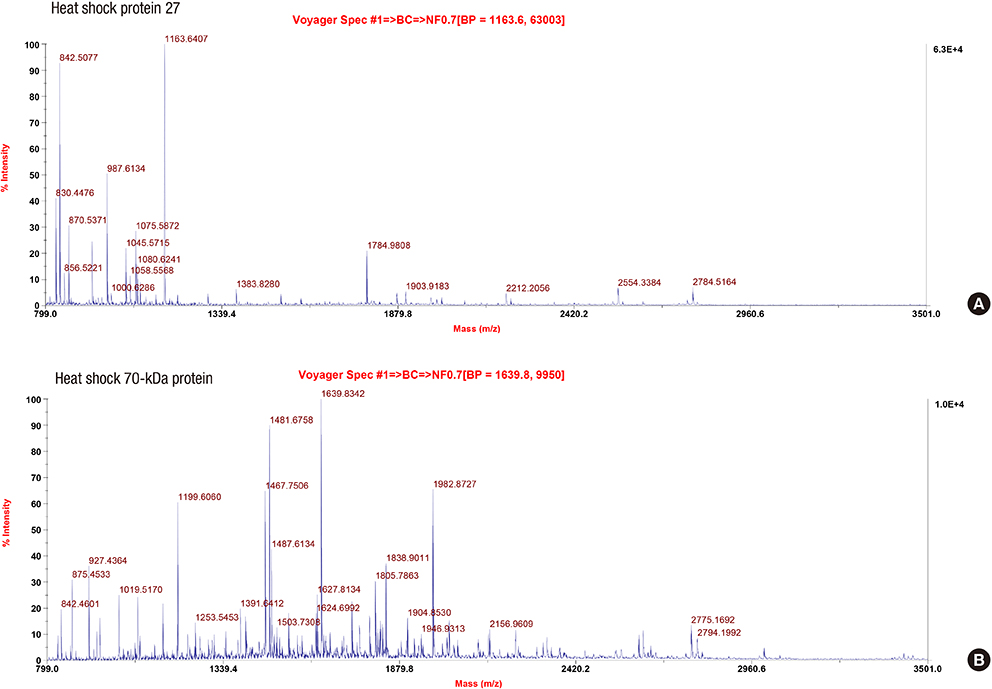
- Preeclampsia is one of the most important and complexed disorders for women's health. Searching for novel proteins as biomarkers to reveal pathogenesis, proteomic approaches using 2DE has become a valuable tool to understanding of preeclampsia. To analyze the proteomic profiling of preclamptic placenta compared to that of normal pregnancy for better understanding of pathogenesis in preeclampsia, placentas from each group were handled by use of proteomics approach using 2DE combined with MALDI-TOF-MS. The 20 spots of showing differences were analysed and identified. Among differentially expressed protein spots Hsp 27 and Hsp 70 were selected for validation using Western blot analysis. In preeclamptic placenta 9 differentially expressed proteins were down-regulated with Hsp 70, serum albumin crystal structure chain A, lamin B2, cytokeratin 18, actin cytoplasmic, alpha fibrinogen precursor, septin 2, dihydrolipoamide branched chain transacylase E2 and firbrinogen beta chain. The 11 up-regulated proteins were fibrinogen gamma, cardiac muscle alpha actin proprotein, cytokeratin 8, calumenin, fibrinogen fragment D, F-actin capping protein alpha-1 subunit, Hsp 27, Hsp 40, annexin A4, enoyl-CoA delta isomerase and programmed cell death protein 6. The western blot analysis for validation also showed significant up-regulation of Hsp 27 and down-regulation of Hsp 70 in the placental tissues with preeclmaptic pregnancies. This proteomic profiling of placenta using 2DE in preeclampsia successfully identifies various proteins involved in apoptosis, mitochondrial dysfunction, as well as three Hsps with altered expression, which might play a important role for the understanding of pathogenesis in preeclampsia.
Keyword
MeSH Terms
Figure
Reference
-
1. Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993; 341:1447–1451.2. Chua S, Wilkins T, Sargent I, Redman C. Trophoblast deportation in pre-eclamptic pregnancy. Br J Obstet Gynaecol. 1991; 98:973–979.3. Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997; 99:2152–2164.4. Hawfield A, Freedman BI. Pre-eclampsia: the pivotal role of the placenta in its pathophysiology and markers for early detection. Ther Adv Cardiovasc Dis. 2009; 3:65–73.5. Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009; 30:S32–S37.6. Kenyon GL, DeMarini DM, Fuchs E, Galas DJ, Kirsch JF, Leyh TS, Moos WH, Petsko GA, Ringe D, Rubin GM, et al. National Research Council Steering Committee. Defining the mandate of proteomics in the post-genomics era: workshop report. Mol Cell Proteomics. 2002; 1:763–780.7. Shankar R, Cullinane F, Brennecke SP, Moses EK. Applications of proteomic methodologies to human pregnancy research: a growing gestation approaching delivery? Proteomics. 2004; 4:1909–1917.8. Hoang VM, Foulk R, Clauser K, Burlingame A, Gibson BW, Fisher SJ. Functional proteomics: examining the effects of hypoxia on the cytotrophoblast protein repertoire. Biochemistry. 2001; 40:4077–4086.9. Hu R, Jin H, Zhou S, Yang P, Li X. Proteomic analysis of hypoxia-induced responses in the syncytialization of human placental cell line BeWo. Placenta. 2007; 28:399–407.10. Johnstone ED, Sawicki G, Guilbert L, Winkler-Lowen B, Cadete VJ, Morrish DW. Differential proteomic analysis of highly purified placental cytotrophoblasts in pre-eclampsia demonstrates a state of increased oxidative stress and reduced cytotrophoblast antioxidant defense. Proteomics. 2011; 11:4077–4084.11. Sun LZ, Yang NN, De W, Xiao YS. Proteomic analysis of proteins differentially expressed in preeclamptic trophoblasts. Gynecol Obstet Invest. 2007; 64:17–23.12. Shi Z, Long W, Zhao C, Guo X, Shen R, Ding H. Comparative proteomics analysis suggests that placental mitochondria are involved in the development of pre-eclampsia. PLoS One. 2013; 8:e64351.13. Kim YN, Kim HK, Warda M, Kim N, Park WS, Prince Adel B, Jeong DH, Lee DS, Kim KT, Han J. Toward a better understanding of preeclampsia: Comparative proteomic analysis of preeclamptic placentas. Proteomics Clin Appl. 2007; 1:1625–1636.14. Shin JK, Baek JC, Kang MY, Park JK, Lee SA, Lee JH, Choi WS, Paik WY. Proteomic analysis reveals an elevated expression of heat shock protein 27 in preeclamptic placentas. Gynecol Obstet Invest. 2011; 71:151–157.15. Wang F, Shi Z, Wang P, You W, Liang G. Comparative proteome profile of human placenta from normal and preeclamptic pregnancies. PLoS One. 2013; 8:e78025.16. Jin H, Ma KD, Hu R, Chen Y, Yang F, Yao J, Li XT, Yang PY. Analysis of expression and comparative profile of normal placental tissue proteins and those in preeclampsia patients using proteomic approaches. Anal Chim Acta. 2008; 629:158–164.17. Gharesi-Fard B, Zolghadri J, Kamali-Sarvestani E. Proteome differences of placenta between pre-eclampsia and normal pregnancy. Placenta. 2010; 31:121–125.18. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000; 183:S1–S22.19. Park KS, Kim H, Kim NG, Cho SY, Choi KH, Seong JK, Paik YK. Proteomic analysis and molecular characterization of tissue ferritin light chain in hepatocellular carcinoma. Hepatology. 2002; 35:1459–1466.20. Choi BK, Cho YM, Bae SH, Zoubaulis CC, Paik YK. Single-step perfusion chromatography with a throughput potential for enhanced peptide detection by matrix-assisted laser desorption/ ionization-mass spectrometry. Proteomics. 2003; 3:1955–1961.21. Ellis J. Proteins as molecular chaperones. Nature. 1987; 328:378–379.22. Brodsky JL, Chiosis G. Hsp70 molecular chaperones: emerging roles in human disease and identification of small molecule modulators. Curr Top Med Chem. 2006; 6:1215–1225.23. Wieten L, Broere F, van der Zee R, Koerkamp EK, Wagenaar J, van Eden W. Cell stress induced HSP are targets of regulatory T cells: a role for HSP inducing compounds as anti-inflammatory immuno-modulators? FEBS Lett. 2007; 581:3716–3722.24. Hnat MD, Meadows JW, Brockman DE, Pitzer B, Lyall F, Myatt L. Heat shock protein-70 and 4-hydroxy-2-nonenal adducts in human placental villous tissue of normotensive, preeclamptic and intrauterine growth restricted pregnancies. Am J Obstet Gynecol. 2005; 193:836–840.25. Ishioka S, Ezaka Y, Umemura K, Hayashi T, Endo T, Saito T. Proteomic analysis of mechanisms of hypoxia-induced apoptosis in trophoblastic cells. Int J Med Sci. 2007; 4:36–44.26. Abdulsid A, Hanretty K, Lyall F. Heat shock protein 70 expression is spatially distributed in human placenta and selectively upregulated during labor and preeclampsia. PLoS One. 2013; 8:e54540.27. Webster RP, Pitzer BA, Roberts VH, Brockman D, Myatt L. Differences in the proteome profile in placenta from normal term and preeclamptic preterm pregnancies. Proteomics Clin Appl. 2007; 1:446–456.28. Luo Q, Jiang Y, Jin M, Xu J, Huang HF. Proteomic analysis on the alteration of protein expression in the early-stage placental villous tissue of electromagnetic fields associated with cell phone exposure. Reprod Sci. 2013; 20:1055–1061.29. Bahnson BJ, Anderson VE, Petsko GA. Structural mechanism of enoyl-CoA hydratase: three atoms from a single water are added in either an E1cb stepwise or concerted fashion. Biochemistry. 2002; 41:2621–2629.30. Rakheja D, Bennett MJ, Foster BM, Domiati-Saad R, Rogers BB. Evidence for fatty acid oxidation in human placenta, and the relationship of fatty acid oxidation enzyme activities with gestational age. Placenta. 2002; 23:447–450.31. Rothhut B. Participation of annexins in protein phosphorylation. Cell Mol Life Sci. 1997; 53:522–526.32. Masuda J, Takayama E, Satoh A, Ida M, Shinohara T, Kojima-Aikawa K, Ohsuzu F, Nakanishi K, Kuroda K, Murakami M, et al. Levels of annexin IV and V in the plasma of pregnant and postpartum women. Thromb Haemost. 2004; 91:1129–1136.33. Sohma H, Ohkawa H, Hashimoto E, Sakai R, Saito T. Ethanol-induced augmentation of annexin IV expression in rat C6 glioma and human A549 adenocarcinoma cells. Alcohol Clin Exp Res. 2002; 26:44s–48s.34. Schoonbroodt S, Piette J. Oxidative stress interference with the nuclear factor-kappa B activation pathways. Biochem Pharmacol. 2000; 60:1075–1083.35. Jeon YJ, Kim DH, Jung H, Chung SJ, Chi SW, Cho S, Lee SC, Park BC, Park SG, Bae KH. Annexin A4 interacts with the NF-kappaB p50 subunit and modulates NF-kappaB transcriptional activity in a Ca2+-dependent manner. Cell Mol Life Sci. 2010; 67:2271–2281.36. Leonard JV, Hyland K, Furukawa N, Clayton PT. Mitochondrial phosphoenolpyruvate carboxykinase deficiency. Eur J Pediatr. 1991; 150:198–199.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gene Expression of Vascular Endothelial Growth Factor(VEGF) and Placental Growth Factor(PlGF) in Human Placenta
- The role of placental indoleamine 2,3-dioxygenase in human pregnancy
- Apoptotic Change in Placenta of Pregnancy-induced Hypertension
- Differential Expression of Placenta Growth Factors and Their Receptors In the Normal and Pregnancy-Induced Hypertensive Human Placentas
- Plasmin System in Placenta from Women with Normal and Preeclamptic Pregnancy