Yonsei Med J.
2007 Aug;48(4):694-700. 10.3349/ymj.2007.48.4.694.
Prognostic Implications of Cyclin B1, p34cdc2, p27(Kip1) and p53 Expression in Gastric Cancer
- Affiliations
-
- 1Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, 108 Pyung-dong, Jongro-gu, Seoul 110-746, Korea. idavidkim@yahoo.co.kr
- KMID: 2158177
- DOI: http://doi.org/10.3349/ymj.2007.48.4.694
Abstract
- PURPOSE
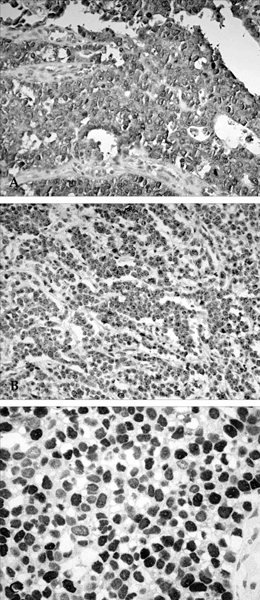
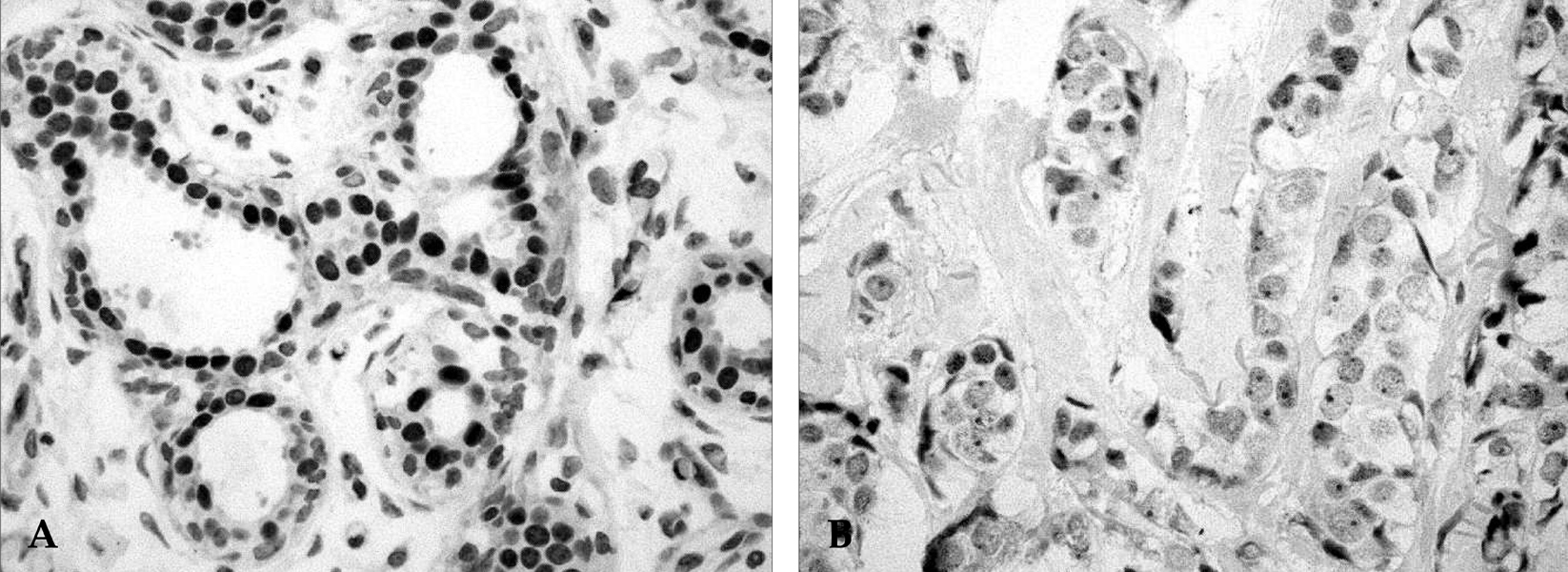
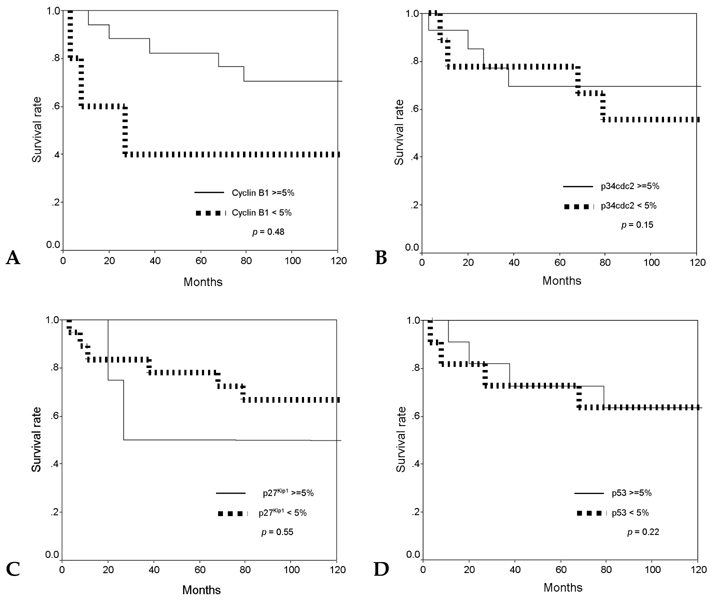
Cell cycle progression is regulated by interactions of specific cyclins and cyclin dependent kinases (CDKs) at the G1-S and G2-M checkpoints and cell cycle deregulation plays a major role in carcinogenesis of human cancers. PATIENTS AND METHODS: To investigate the role of cell cycle regulators in the pathogenesis and progression of human gastric cancers, 23 cases of gastric carcinomas were examined for the expression of cyclin B1, p34cdc2, p27(Kip1) and p53 by immunohistochemical methods, and gene expression was correlated with various clinicopathologic findings. RESULTS: Out of 23 cases studied, cyclin B1 was diffusely expressed in 20 cases (87.0%), p34cdc2 in 14 cases (60.9%) and p53 in 12 cases (52.2%), whereas in normal gastric tissues, cyclin B1 and p34cdc2 were weakly expressed and p53 was not expressed. In contrast, p27(Kip1) was expressed in only 8.7% of gastric carcinomas compared with 78.3% of normal gastric tissues. There was correlation between the expression of cyclin B1 and expression of p34cdc2 (p=0.002), between the expression of cyclin B1 and loss of p27(Kip1) (p=0.025), and between the expression of p34cdc2 and loss of p27(Kip1) (p=0.065). In addition, expression of cyclin B1 was correlated with regional lymph node metastasis (p=0.032). CONCLUSION: Our results indicate that cyclin B1 and p34cdc2 are involved in the genesis or progression of gastric cancers. Furthermore, overexpression of cyclin B1 may play an important role in lymph node metastatic potential of gastric cancer. Thus, abnormal expression of cyclin B1 and CDKs, overexpression of p53 and loss of p27(Kip1) expression may play important roles in human gastric carcinogenesis.
Keyword
MeSH Terms
Figure
Reference
-
1. Hunter T, Pines J. Cyclins and cancer. Cell. 1991. 66:1071–1074.
Article2. Nurse P. Ordering S phase and M phase in the cell cycle. Cell. 1994. 79:547–550.
Article3. Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995. 9:1149–1163.
Article4. Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994. 266:1821–1828.
Article5. Ohta T, Okamoto K, Isohashi F, Shibata K, Fukuda M, Yamaguchi S, et al. T-loop deletion of CDC2 from breast cancer tissues eliminates binding to cyclin B1 and cyclin-dependent kinase inhibitor p21. Cancer Res. 1998. 58:1095–1098.6. Nakayama K, Nakayama K. Cip/Kip cyclin-dependent kinase inhibitors: brakes of the cell cycle engine during development. Bioessays. 1998. 29:1020–1029.
Article7. Catzavelos C, Bhattacharya N, Ung YC, Wilson JA, Roncari L, Sandhu C, et al. Decreased levels of the cell- cyclin inhibitor p27kip1 protein: prognostic implications in primary breast cancer. Nat Med. 1997. 3:227–230.
Article8. Imamura J, Miyoshi I, Koeffler HP. p53 in hematologic malignancies. Blood. 1994. 84:2412–2421.
Article9. American Joint Committee on Cancer. AJCC Cancer Staging Manual. 2002. 6th ed. New York: Springer-Verlag;99–103.10. Wattel E, Preudhomme C, Hecquet B, Vanrumbeke M, Quesnel B, Dervite I, et al. p53 mutations are associated with resistance to chemotherapy and short survival in hematologic malignancies. Blood. 1994. 84:3148–3157.
Article11. Pines J, Hunter T. Human cyclin A is adenovirus E1A- associated protein p60 and behaves differently from cyclin B. Nature. 1990. 346:760–763.
Article12. Murakami H, Furihata M, Ohtsuki Y, Ogoshi S. Determination of the prognostic significance of cyclin B1 overexpression in patients with esophageal squamous cell carcinoma. Virchows Arch. 1999. 434:153–158.
Article13. Soria JC, Jang SJ, Khuri FR, Hassan K, Liu D, Hong WK, et al. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res. 2000. 60:4000–4004.14. Ito Y, Takeda T, Sakon M, Monden M, Tsujimoto M, Matsuura N. Expression and prognostic role of cyclin-dependent kinase 1(cdc2) in hepatocellular carcinoma. Oncology. 2000. 59:68–74.
Article15. Park YJ, Wen J, Bang S, Park SW, Song SY. [6]-Gingerol induces cell cycle arrest and cell death of mutant p53-expressing pancreatic cancer cells. Yonsei Med J. 2006. 47:688–697.
Article16. Jeong J, Park YN, Park JS, Yoon DS, Chi HS, Kim BR. Clinical significance of p16 protein expression loss and aberrant p53 protein expression in pancreatic cancer. Yonsei Med J. 2005. 46:519–525.
Article17. Fernández-Figueras MT, Casalots A, Puig L, Llatjós R, Ferrándiz C, Ariza A. Proliferating trichilemmal tumour: p53 immunoreactivity in association with p27Kip1 over-expression indicates a low-grade carcinoma profile. Histopathology. 2000. 38:454–457.
Article18. Baker SJ, Fearon ER, Nigro JM, Hamilton SR, Preisinger AC, Jessup JM, et al. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989. 244:217–221.
Article19. Shaw PH. The role of p53 in cell cycle regulation. Pathol Res Pract. 1996. 192:669–675.
Article20. Vogelstein B, Kinzler KW. p53 function and dysfunction. Cell. 1992. 70:523–526.
Article21. Lee PL, Kim JS, Yeon HC, Lee YC, Lee SU, Kim DW, et al. Correlation between p53 and p27kip1 expression and clinicopathologic features in hepatocellular carcinoma. J Korean Cancer Assoc. 2000. 32:390–397.22. Craig C, Wersto R, Kim M, Ohri E, Li Z, Katayose D, et al. A recombinant adenovirus expressing p27kip1 induces cell cycle arrest and loss of cyclin-Cdk activity in human breast cancer cells. Oncogene. 1997. 14:2283–2289.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of p27kip1, Cyclin D1 and p53 Protein in Ductal Carcinoma In Situ of the Breast
- Expression of p34(cdc2), p27(Kip1), p21(WAF1/Cip1) and p53 in Human Breast Cancers
- Prognostic Significance of p27(kip1) Expression in Node Negative Colorectal Carcinoma
- Significance of the Expression of p27(Kip1) Protein in Human Breast Cancer
- Expression of p27(Kip1) in gastric cancers and precancerous lesions