Yonsei Med J.
2005 Feb;46(1):119-124. 10.3349/ymj.2005.46.1.119.
Tumor Volume Change after Chemotheraphy as a Predictive Factor of Disease Free Survival for Osteosarcoma
- Affiliations
-
- 1Department of Orthopaedic Surgery, Yonsei University College of Medicine, Seoul, Korea. qshin@yumc.yonsei.ac.kr
- 2Department of Diagnostic Radiology, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 4Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2158121
- DOI: http://doi.org/10.3349/ymj.2005.46.1.119
Abstract
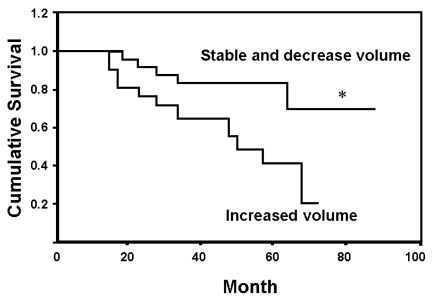
- Change in tumor volume after chemotherapy appears to have a prognostic significance for the outcome of osteosarcoma. A newly developed volume measurement method based on three-dimensional summation with a proved reproducibility was utilized to measure osteosarcoma tumor volume. This retrospective analysis included 38 patients with biopsy- proven, nonsurface, skeletal high-grade osteosarcoma. The treatment was started by using three cycles of preoperative chemotherapy with cisplastin (100 mg/m2) and adriamycin (30 mg/m2). The tumor volume was measured before and after preoperative chemotherapy using three-dimensional magnetic resonance image measurement. The percentage of tumor necrosis was assessed by pathologic exam. After three cycle of postoperative chemotherapy, the patients were followed up at regular interval. For the 23 good responder patients, the mean survival time was 73.2 months (95% confidence interval 61.9 - 84.5 months), and for the 15 poor responder patients, the mean survival time was 50.8 months (95% confidence interval 38.6 - 63.1 months) (p < 0.05). For the 14 patients with increased tumor volume after chemotherapy, the mean survival time was 47.5 months (range: 36.3 - 58.6 months) and for the 24 patients with stable or decreased tumor volume, the mean survival time was 74.3 months (range: 63.79 - 84.88 months) (p < 0.05). Among the various factors, histopathologic response and tumor volume change after chemotherapy predicted disease free survival (p < 0.05). Change in the tumor volume that was measured with a reproducible method and the histopathologic response after chemotherapy were the important predictors of disease free survival for osteosarcoma patients.
Keyword
MeSH Terms
-
Antibiotics, Antineoplastic/therapeutic use
Antineoplastic Agents/therapeutic use
Bone Neoplasms/drug therapy/*mortality/*pathology
Cisplatin/therapeutic use
Disease-Free Survival
Doxorubicin/therapeutic use
Humans
Osteosarcoma/drug therapy/*mortality/*pathology
Predictive Value of Tests
Prognosis
Retrospective Studies
Figure
Reference
-
1. Glasser DB, Lane JM, Huvos AG, Marcove RC, Rosen G. Survival, prognosis, and therapeutic response in osteogenic sarcoma. Cancer. 1992; 69:698–708. PMID: 1730120.2. Bacci G, Ferrari S, Delepine N, Bertoni F, Picci P, Mercuri M, et al. Predictive factors of histologic response to primary chemotherapy in osteosarcoma of the extremity: Study of 272 patients preoperatively treated with high-dose methotrexate, doxorubicin, and cisplatin. J Clin Oncol. 1998; 16:658–663. PMID: 9469355.
Article3. Goorin AM, Abelson HT, Frei E III. Osteosarcoma: Fifteen years later. N Engl J Med. 1985; 313:1637–1641. PMID: 3906399.
Article4. Rosen G, Caparros B, Huvos AG, Kosloff C, Nirenberg A, Cacavio A, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982; 49:1221–1230. PMID: 6174200.5. Rosen G, Murphy ML, Huvos AG, Gutierrez M, Marcove RC. Chemotherapy, en bloc resection and prosthetic bone replacement in the treatment of osteogenic sarcoma. Cancer. 1976; 37:1–11. PMID: 1082364.6. Bieling P, Rehan N, Winkler P, Helmke K, Maas R, Fuchs N, et al. Tumor size and prognosis in aggressively treated osteosarcoma. J Clin Oncol. 1996; 14:848–858. PMID: 8622033.
Article7. Bronstrom LA, Strander H, Nilsonne U. Survival on osteosarcoma in relation to tumor size and location. Clin Orthop. 1982; 167:250–254. PMID: 6954021.8. Hudson M, Jaffe R, Jaffe N, Ayala A, Raymond AK, Carrasco H, et al. Pediatric osteosarcoma: Therapeutic strategies, results, and prognostic factors derived from a 10-year experience. J Clin Oncol. 1990; 8:1988–1997. PMID: 2230890.
Article9. Meyers PA, Heller G, Healey J, Huvos A, Lane J, Marcove R, et al. Chemotherapy for nonmetastatic osteosarcoma. The Memorial Sloan-Kettering experience. J Clin Oncol. 1992; 10:5–15. PMID: 1370176.10. Pochanugool L, Subhadharaphandou T, Dhanachai M, Hathirat P, Sangthawan D, Pirabul R, et al. Prognostic factor among 130 patients with osteosarcoma. Clin Orthop. 1997; 345:206–214. PMID: 9418642.11. Scranton P, DeCicco F, Totten T, Yunis EJ. Prognostic factors in osteosarcoma: A review of 20 years experience at the University of Pittsburgh Health Center hospital. Cancer. 1975; 36:2179–2191. PMID: 1060506.12. Taylor WF, Ivins JC, Dahlin DC, Edmonson JH, Pritchard DJ, et al. Trends and variability in survival from osteosarcoma. Mayo Clin Proc. 1978; 53:695–700. PMID: 280739.13. Holscher HC, Bloem JL, Van der Woude HJ, Hermans J, Nooy MA, Taminiau AH, et al. Can MRI predict the histopathologic response in patients with osteosarcoma after the first cycle of chemotherapy? Clin Radiol. 1995; 50:384–390. PMID: 7789022.14. Shin KH, Moon SH, Suh JS, Yang WI. Tumor volume change as a predictor of chemotherapeutic response in osteosarcoma. Clin Orthop. 2000; 376:200–208. PMID: 10906876.
Article15. Enneking WF, Spanier SS, Goodman M. Current concepts review: Surgical staging of musculoskeletal sarcoma. J Bone Joint Surg Am. 1980; 62:1027–1030. PMID: 7000786.16. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916 (classical article). Nutrition. 1989; 5:303–311. PMID: 2520314.17. Huvos AG, Rosen G, Marcove RC. Primary osteogenic sarcoma: Pathologic aspects in 20 patients after treatment with chemotherapy, en bloc resection, and prosthetic bone replacement. Arch Pathol Lab Med. 1977; 101:14–18. PMID: 299812.18. Erlemann R, Sciuk J, Bosse A, Ritter J, Kusnierz-Glaz CR, Peters PE, et al. Response of osteosarcoma and Ewing sarcoma to preoperative chemotherapy: Assessment with dynamic and static MR imaging and skeletal scintigraphy. Radiology. 1990; 175:791–796. PMID: 2188300.
Article19. Holscher HC, Bloem JL, Nooy MA, Taminiau AH, Eulderink F, Hermans J, et al. The value of MR imaging in monitoring the effect of chemotherapy on bone sarcoma. Am J Roentgenol. 1990; 154:763–769. PMID: 2107673.20. Welling RM, Davies AM, Pynsent PB, Carter SR, Grimer RJ. The value of computed tomographic measurements in osteosarcoma as a predictor of response to adjuvant chemotherapy. Clin Radiol. 1994; 49:19–23. PMID: 8299327.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Osteosarcoma in Korean children and adolescents
- Development of Conventional Osteosarcoma after 13 Years Continuous Disease-free Survival of Periosteal Osteosarcoma
- Distal Radius Osteosarcoma
- The analysis of prognostic factors in patients with epithelial ovarian cancer
- Prognostic Factors and Outcome in Childhood and Adolescent Osteosarcoma: Single Institution Experience