J Korean Med Sci.
2010 Oct;25(10):1513-1517. 10.3346/jkms.2010.25.10.1513.
The Common NF-kappaB Essential Modulator (NEMO) Gene Rearrangement in Korean Patients with Incontinentia Pigmenti
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. changski@skku.edu
- 2Department of Pediatrics, Seoul National University School of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Ewha Womans University School of Medicine, Seoul, Korea.
- KMID: 2157858
- DOI: http://doi.org/10.3346/jkms.2010.25.10.1513
Abstract
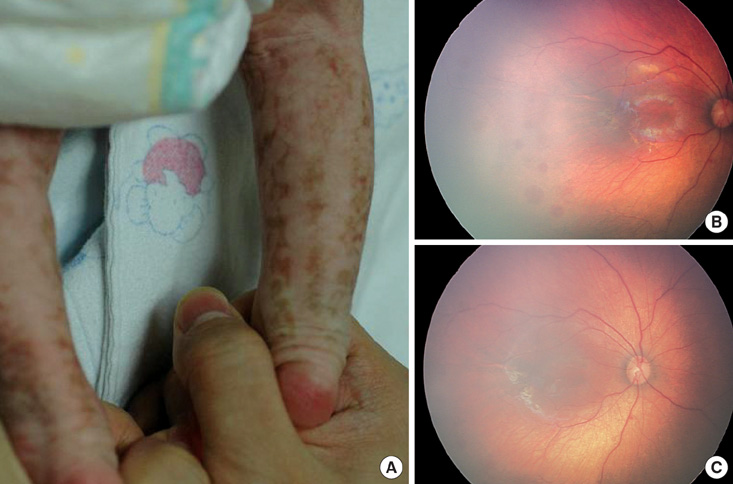
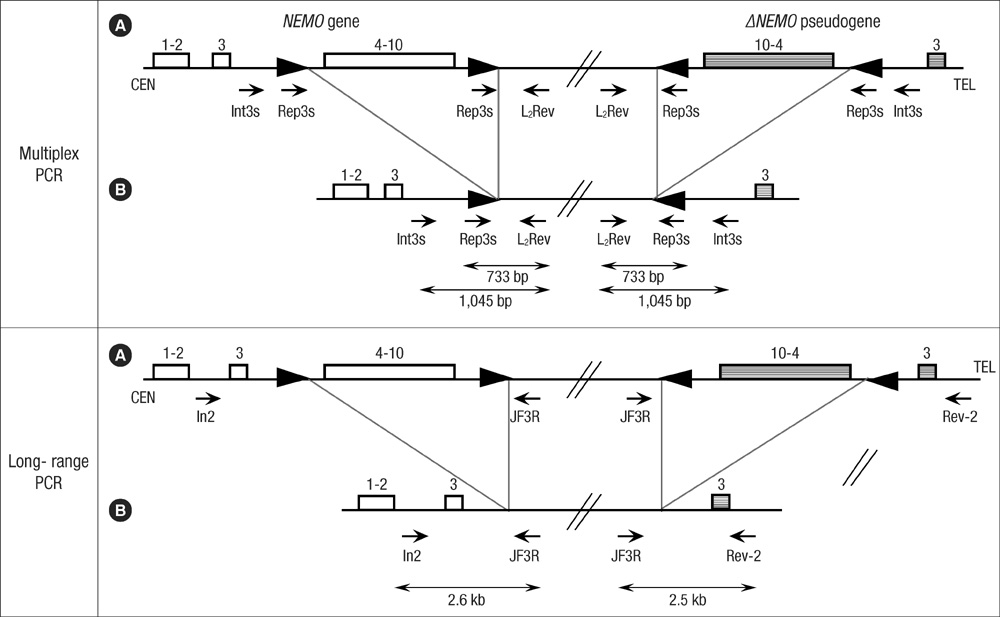
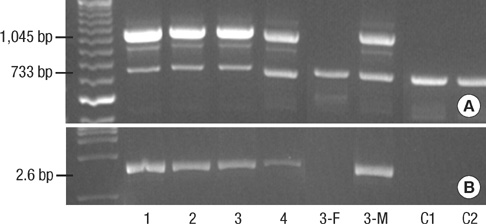
- Incontinentia pigmenti (IP) is a rare X-linked dominant disorder characterized by highly variable abnormalities of the skin, eyes and central nervous system. A mutation of the nuclear factor-kappa B essential modulator (NEMO) located at Xq28 is believed to play a role in pathogenesis and the mutation occurs mostly in female patients due to fatal consequence of the mutation in males in utero. This study was designed to identify the common NEMO rearrangement in four Korean patients with IP. Deletion of exons 4 to 10 in the NEMO, the most common mutation in IP patients, was detected in all of the patients by the use of long-range PCR analysis. This method enabled us to discriminate between NEMO and pseudogene rearrangements. Furthermore, all of the patients showed skewed XCI patterns, indicating pathogenicity of IP was due to cells carrying the mutant X chromosome. This is the first report of genetically confirmed cases of IP in Korea.
Keyword
MeSH Terms
Figure
Reference
-
1. Berlin AL, Paller AS, Chan LS. Incontinentia pigmenti: a review and update on the molecular basis of pathophysiology. J Am Acad Dermatol. 2002. 47:169–187.
Article2. Landy SJ, Donnai D. Incontinentia pigmenti (Bloch-Sulzberger syndrome). J Med Genet. 1993. 30:53–59.
Article3. Parrish JE, Scheuerle AE, Lewis RA, Levy ML, Nelson DL. Selection against mutant alleles in blood leukocytes is a consistent feature in Incontinentia Pigmenti type 2. Hum Mol Genet. 1996. 5:1777–1783.
Article4. Aradhya S, Courtois G, Rajkovic A, Lewis RA, Levy M, Israel A, Nelson DL. Atypical forms of incontinentia pigmenti in male individuals result from mutations of a cytosine tract in exon 10 of NEMO (IKK-gamma). Am J Hum Genet. 2001. 68:765–771.5. Smahi A, Courtois G, Vabres P, Yamaoka S, Heuertz S, Munnich A, Israel A, Heiss NS, Klauck SM, Kioschis P, Wiemann S, Poustka A, Esposito T, Bardaro T, Gianfrancesco F, Ciccodicola A, D'Urso M, Woffendin H, Jakins T, Donnai D, Stewart H, Kenwrick SJ, Aradhya S, Yamagata T, Levy M, Lewis RA, Nelson DL. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. The International Incontinentia Pigmenti (IP) Consortium. Nature. 2000. 405:466–472.6. Fusco F, Bardaro T, Fimiani G, Mercadante V, Miano MG, Falco G, Israel A, Courtois G, D'Urso M, Ursini MV. Molecular analysis of the genetic defect in a large cohort of IP patients and identification of novel NEMO mutations interfering with NF-kappaB activation. Hum Mol Genet. 2004. 13:1763–1773.7. Aradhya S, Woffendin H, Jakins T, Bardaro T, Esposito T, Smahi A, Shaw C, Levy M, Munnich A, D'Urso M, Lewis RA, Kenwrick S, Nelson DL. A recurrent deletion in the ubiquitously expressed NEMO (IKK-gamma) gene accounts for the vast majority of incontinentia pigmenti mutations. Hum Mol Genet. 2001. 10:2171–2179.
Article8. Steffann J, Raclin V, Smahi A, Woffendin H, Munnich A, Kenwrick SJ, Grebille AG, Benachi A, Dumez Y, Bonnefont JP, Hadj-Rabia S. A novel PCR approach for prenatal detection of the common NEMO rearrangement in incontinentia pigmenti. Prenat Diagn. 2004. 24:384–388.
Article9. Bardaro T, Falco G, Sparago A, Mercadante V, Gean Molins E, Tarantino E, Ursini MV, D'Urso M. Two cases of misinterpretation of molecular results in incontinentia pigmenti, and a PCR-based method to discriminate NEMO/IKKgamma dene deletion. Hum Mutat. 2003. 21:8–11.10. Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992. 51:1229–1239.11. Xinhua B, Shengling J, Fuying S, Hong P, Meirong L, Wu XR. X chromosome inactivation in Rett Syndrome and its correlations with MECP2 mutations and phenotype. J Child Neurol. 2008. 23:22–25.
Article12. Huang J, Kondo H, Uchio E. A case of incontinentia pigmenti in Japan and its genetic examination. Jpn J Ophthalmol. 2007. 51:142–145.
Article13. Tada H, Yoshida S, Yamaji Y, Fujisawa K, Ishibashi T. NEMO mutational analysis in a Japanese family with incontinentia pigmenti. Eye. 2007. 21:888–890.
Article14. Kim BJ, Shin HS, Won CH, Lee JH, Kim KH, Kim MN, Ro BI, Kwon OS. Incontinentia pigmenti: clinical observation of 40 Korean cases. J Korean Med Sci. 2006. 21:474–477.
Article15. Martinez-Pomar N, Munoz-Saa I, Heine-Suner D, Martin A, Smahi A, Matamoros N. A new mutation in exon 7 of NEMO gene: late skewed X-chromosome inactivation in an incontinentia pigmenti female patient with immunodeficiency. Hum Genet. 2005. 118:458–465.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Genetic Study in a Patient with Incontinentia Pigmenti
- A Case of Incontinentia Pigmenti in a Boy with Klinefelter Syndrome
- Incontinentia Pigmenti in a Newborn with NEMO Mutation
- A Case of Incontinentia Pigmenti with Multiple Brain Infarction
- Incontinentia Pigmenti with Extensive Brain Infarction in a Newborn