J Korean Med Sci.
2010 Oct;25(10):1492-1498. 10.3346/jkms.2010.25.10.1492.
GD3 Accumulation in Cell Surface Lipid Rafts Prior to Mitochondrial Targeting Contributes to Amyloid-beta-induced Apoptosis
- Affiliations
-
- 1Department of Neurology, Dong-A University College of Medicine, Medical Science Research Center, Busan, Korea.
- 2Department of Physiology, Dong-A University College of Medicine, Medical Science Research Center, Busan, Korea. hrbae@dau.ac.kr
- KMID: 2157855
- DOI: http://doi.org/10.3346/jkms.2010.25.10.1492
Abstract
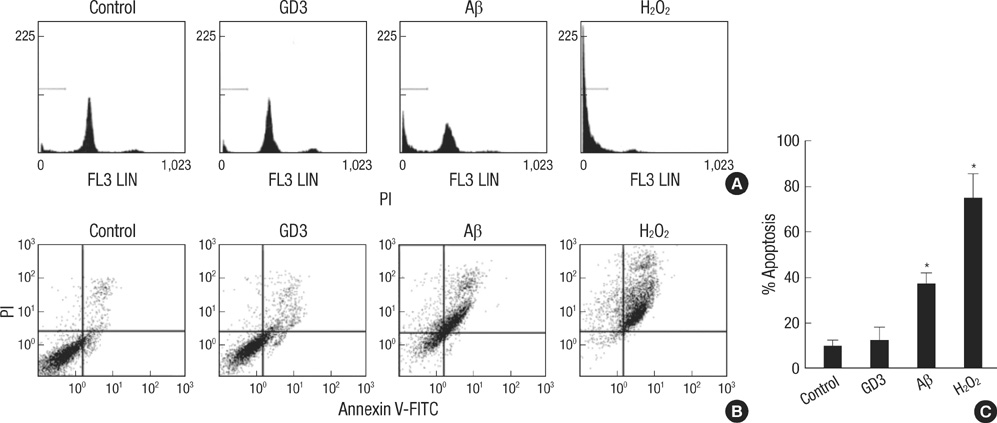
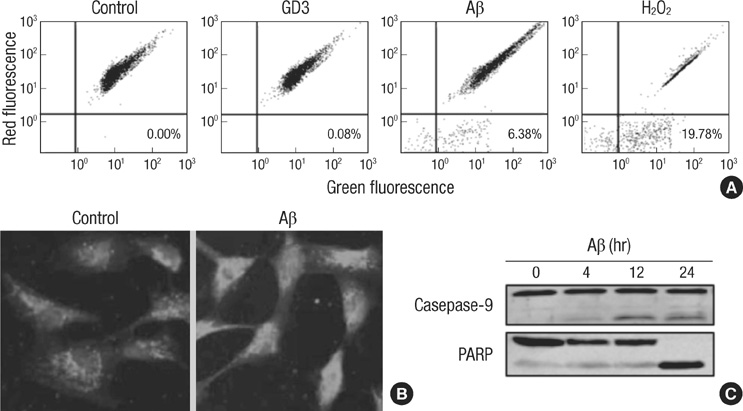
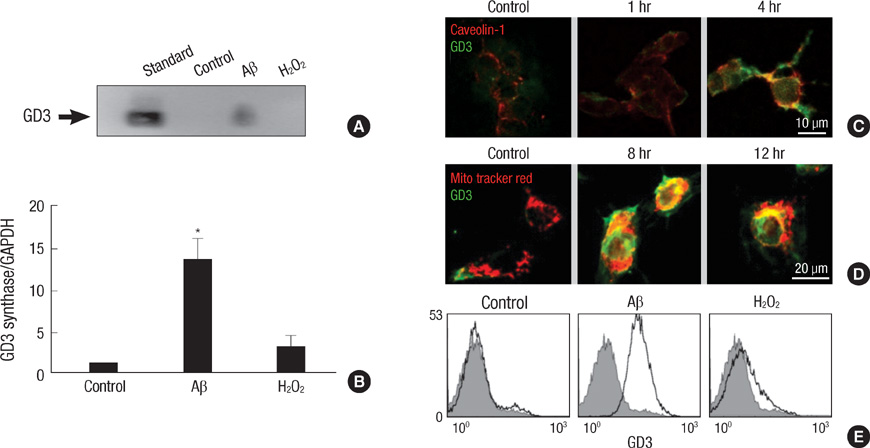
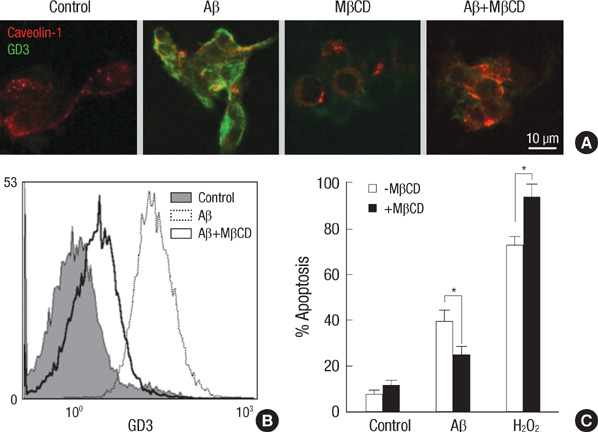
- Neuronal apoptosis induced by amyloid beta-peptide (A beta) plays an important role in the pathophysiology of Alzheimer's disease (AD). However, the molecular mechanism underlying A beta-induced apoptosis remains undetermined. The disialoganglioside GD3 involves ceramide-, Fas- and TNF-alpha-mediated apoptosis in lymphoid cells and hepatocytes. Although the implication of GD3 has been suggested, the precise role of GD3 in A beta-induced apoptosis is still unclear. Here, we investsigated the changes of GD3 metabolism and characterized the distribution and trafficking of GD3 during A beta-induced apoptosis using human brain-derived TE671 cells. Extracellular A beta induced apoptosis in a mitochondrial-dependent manner. GD3 level was negligible in the basal condition. However, in response to extracellular A beta, both the expression of GD3 synthase mRNA and the intracellular GD3 level were dramatically increased. Neosynthesized GD3 rapidly accumulated in cell surface lipid microdomains, and was then translocated to mitochondria to execute the apoptosis. Disruption of membrane lipid microdomains with methyl-beta-cyclodextrin significantly prevented both GD3 accumulation in cell surface and A beta-induced apoptosis. Our data suggest that rapidly accumulated GD3 in plasma membrane lipid microdomains prior to mitochondrial translocation is one of the key events in A beta-induced apoptosis.
MeSH Terms
Figure
Reference
-
1. Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004. 430:631–639.
Article2. Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nat Rev Mol Cell Biol. 2007. 8:101–112.
Article3. Hakomori S. Glycosylation defining cancer malignancy: New wine in an old bottle. Proc Natl Acad Sci USA. 2002. 99:10231–10233.
Article4. Lee MC, Lee WS, Park CS, Juhng SW. The biologic role of ganglioside in neuronal differentiation: effects of GM1 ganglioside on human neuroblastoma SH-SY5Y cells. J Korean Med Sci. 1994. 9:179–187.
Article5. Malisan F, Testi R. GD3 ganglioside and apoptosis. Biochim Biophys Acta. 2002. 1585:179–187.
Article6. De Maria R, Lenti L, Malisan F, d'Agostino F, Tomassini B, Zeuner A, Rippo MR, Testi R. Requirement for GD3 ganglioside in CD95- and ceramide-induced apoptosis. Science. 1997. 277:1652–1655.
Article7. Bektas M, Spiegel S. Glycosphingolipids and cell death. Glycoconj J. 2004. 20:39–47.
Article8. Simon BM, Malisan F, Testi R, Nicotera P, Leist M. Disialoganglioside GD3 is released by microglia and induces oligodendrocyte apoptosis. Cell Death Differ. 2002. 9:758–767.
Article9. Copani A, Melchiorri D, Caricasole A, Martini F, Sale P, Carnevale R, Gradini R, Sortino MA, Lenti L, De Maria R, Nicoletti F. β-Amyloid-induced synthesis of the ganglioside GD3 is a requisite for cell cycle reactivation and apoptosis in neurons. J Neurosci. 2002. 22:3963–3968.
Article10. Synapin PJ, Salvaterra PM, Engelhardt JK. Neuron-like features of TE671 cells: presence of a functional nicotinic cholinergic receptor. Brain Res. 1982. 231:365–377.11. Colell A, Morales A, Fernandez-Checa JC, Garcia-Ruiz C. Ceramide generated by acidic sphingomyelinase contributes to tumor necrosis factor-alpha-mediated apoptosis in human colon HT-29 cells through glycosphingolipids formation. Possible role of ganglioside GD3. FEBS Lett. 2002. 526:135–141.12. Garcia-Ruiz C, Colell A, Morales A, Calvo M, Enrich C, Fernández-Checa JC. Trafficking of ganglioside GD3 to mitochondria by tumor necrosis factor-alpha. J Biol Chem. 2002. 277:36443–36448.13. Kristal BS, Brown AM. Apoptogenic ganglioside GD3 directly induces the mitochondrial permeability transition. J Biol Chem. 1999. 274:23169–23175.
Article14. Tomassini B, Testi R. Mitochondria as sensors of sphingolipids. Biochimie. 2002. 84:123–129.
Article15. Morales A, Colell A, Mari M, Garcia-Ruiz C, Fernandez-Checa JC. Glycosphingolipids and mitochondria: role in apoptosis and disease. Glycoconj J. 2004. 20:579–588.
Article16. Garcia-Ruiz C, Colell A, Paris R, Fernandez-Checa JC. Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. FASEB J. 2000. 14:847–858.17. Malisan F, Franchi L, Tomassini B, Ventura N, Condo I, Rippo MR, Rufini A, Liberati L, Nachtigall C, Kniep B, Testi R. Acetylation suppresses the proapoptotic activity of GD3 ganglioside. J Exp Med. 2002. 196:1535–1541.
Article18. Brenner C, Kniep B, Maillier E, Martel C, Franke C, Röber N, Bachmann M, Rieber EP, Sandhoff R. GD3-7-aldehyde is an apoptosis inducer and interacts with adenine nucleotide translocase. Biochem Biophys Res Commun. 2010. 391:248–253.
Article19. Ariga T, McDonald MP, Yu RK. Role of ganglioside metabolism in the pathogenesis of Alzheimer's disease - a review. J Lipid Res. 2008. 49:1157–1175.20. Miyagi T, Wada T, Yamaguchi K, Hata K, Shiozaki K. Plasma membrane-associated sialidase as a crucial regulator of transmembrane signaling. J Biochem. 2008. 144:279–285.21. Hasegawa T, Sugeno N, Takeda A, Matsuzaki-Kobayashi M, Kikuchi A, Furukawa K, Miyagi T, Itoyama Y. Role of Neu4L sialidase and its substrate ganglioside GD3 in neuronal apoptosis induced by catechol metabolites. FEBS Lett. 2007. 581:406–412.
Article22. Choo-Smith LP, Garzon-Rodriguez W, Glabe CG, Surewicz WK. Acceleration of amyloid fibril formation by specific binding of Abeta-(1-40) peptide to ganglioside-containing membrane vesicles. J Biol Chem. 1997. 272:22987–22990.23. Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000. 1:31–39.
Article24. Patron RG. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J Histochem Cytochem. 1994. 42:155–166.25. Kakio A, Nishimoto S, Kozutsumi Y, Matsuzaki K. Formation of a membrane-active form of amyloid β-protein in raft-like model membranes. Biochem Biophys Res Commun. 2003. 303:514–518.
Article26. Hayashi H, Kimura N, Yamaguchi H, Hasegawa K, Yokoseki T, Shibata M, Yamamoto N, Michikawa M, Yoshikawa Y, Terao K, Matsuzaki K, Lemere CA, Selkoe DJ, Naiki H, Yanagisawa K. A seed for Alzheimer amyloid in the brain. J Neurosci. 2004. 24:4894–4902.
Article27. Vyas KA, Patel HV, Vyas AA, Schnaar RL. Segregation of gangliosides GM1 and GD3 on cell membranes, isolated membrane rafts, and defined supported lipid monolayers. Biol Chem. 2001. 382:241–250.
Article28. Bernardo A, Harrison FE, McCord M, Zhao J, Bruchey A, Davies SS, Jackson Roberts L 2nd, Mathews PM, Matsuoka Y, Ariga T, Yu RK, Thompson R, McDonald MP. Elimination of GD3 synthase improves memory and reduces amyloid-beta plaque load in transgenic mice. Neurobiol Aging. 2009. 30:1777–1791.29. Colell A, Garcia-Ruiz C, Roman J, Ballesta A, Fernandez-Checa JC. Ganglioside GD3 enhances apoptosis by suppressing the nuclear factor-kappa B-dependent survival pathway. FASEB J. 2001. 15:1068–1070.30. Tempera I, Buchetti B, Lococo E, Gradini R, Mastronardi A, Mascellino MT, Sale P, Mosca L, d'Erme M, Lenti L. GD3 nuclear localization after apoptosis induction in HUT-78 cells. Biochem Biophys Res Commun. 2008. 368:495–500.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A1 Receptor-mediated Protection against Amyloid Beta-induced Injury in Human Neuroglioma Cells
- Protective Effect of Fibroin BF-7 on Neuronal Cell Death in Alzheimer Model using Amyloid beta Peptide
- Mercury induced the Accumulation of Amyloid Beta (Abeta) in PC12 Cells: The Role of Production and Degradation of Abeta
- Extracellular ATP is generated by ATP synthase complex in adipocyte lipid rafts
- PC12 Cell Apoptosis Mediated by Reactive Oxygen Species with Various Agents is Reduced Effectively by Flavonoid Antioxidants