J Korean Med Sci.
2006 Feb;21(1):136-142. 10.3346/jkms.2006.21.1.136.
Expression of TGFbeta Family in the Developing Internal Ear of Rat Embryos
- Affiliations
-
- 1Department of Anatomy, College of Medicine, Kwandong University, Gangneung, Korea.
- 2Department of Anatomy, College of Medicine, Seonam University, Namwon, Korea.
- 3Department of Otorhinolaryngology, College of Medicine, Seonam University, Namwon, Korea.
- 4Department of Anatomy, College of Medicine, Kosin University, Busan, Korea. drhkim@kosin.ac.kr
- KMID: 2157797
- DOI: http://doi.org/10.3346/jkms.2006.21.1.136
Abstract
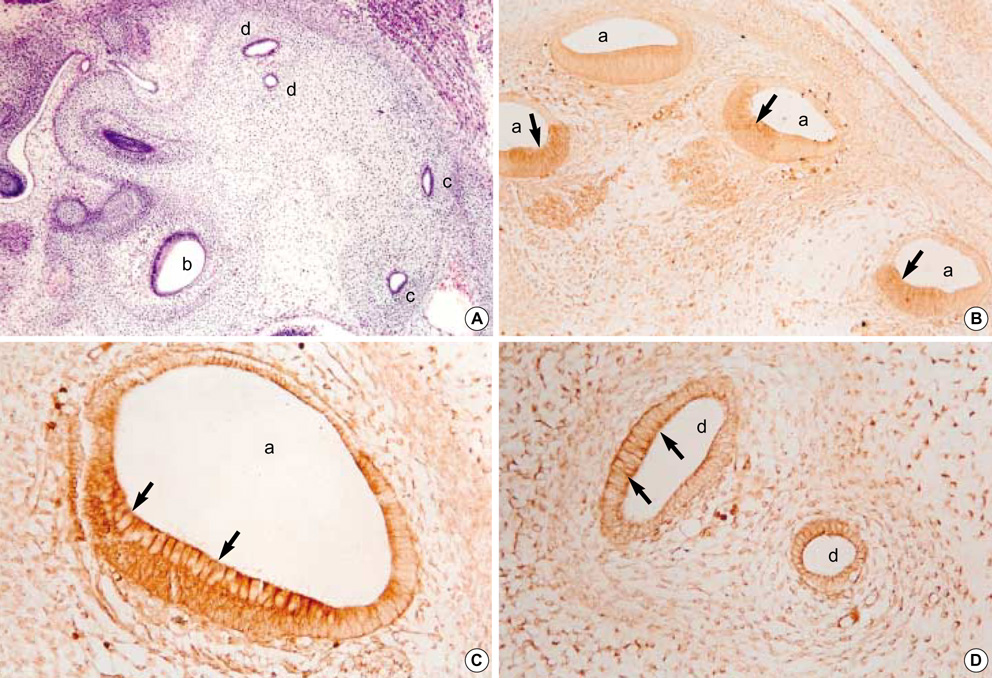
- In order to investigate the expression patterns of the transforming growth factor (TGF)beta isoforms in the internal ear, an immunohistochemical study of rat embryos was performed. Rat embryos were taken on the 13th, 15th, 17th, and 19th day after conception and their internal ears were immunohistochemically stained against TGF beta1, beta2, and beta3. As a result, the 13-day-old embryo showed a very weak positivity to TGF beta1. After the 15th day of pregnancy, no reactivity to TGF beta1 was defected. Immunoreactivity to TGF beta2 was observed from the 15th day of pregnancy throughout the rest of the period. The ampulla of the semicircular canal and the cochlear duct showed a notably strong immunohistochemical reaction. A strong reaction to TGF beta3 was observed on the 15th day of pregnancy. However, no positive reactions were observed thereafter. A strong immunoreactivity was observed especially on the apical cytoplasms, the surfaces of the epithelial cells, and basement membranes of the cochlear duct, as well as the semicircular canals of the developing internal ear of rat embryo.
MeSH Terms
Figure
Reference
-
1. Larsen WJ. Human Embryology. 2001. 3rd Edition. Oxford: Churchill Livingstone;392–412.2. Moore KL, Persaud TV. The Developing Human: Clinically oriented embryology. 2003. 7th Edition. Philadelphia: Saunders;476–479.3. Park HW. Human embryology. 2005. 3rd Edition. Seoul: Koonja Publishing Inc.;503–507.4. Streit A. Origin of the vertebrate inner ear: evolution and induction of the otic placode. J Anat. 2001. 199:99–103.
Article5. O'Rahilly R, Muller F. Developmental Stages in Human Embryos. 1987. Washington DC: Carnegie Institution of Washington;186.6. Theiler K. The House Mouse. 1972. New York: Springer-Verlag;44–108.7. Rugh R. The mouse. Its reproduction and development. 1991. Oxford: Oxford University Press;249–251.8. Miller DA, Pelton RW, Derynck R, Moses HL. Transforming growth factor-beta. A family of growth regulatory peptides. Ann N Y Acad Sci. 1990. 593:208–217.9. McCartney-Francis NL, Frazier-Jessen M, Wahl SM. TGF-beta: a balancing act. Int Rev Immunol. 1998. 16:553–580.10. Akhurst RJ, Lehnert SA, Gatherer D, Duffie E. The role of TGF beta in mouse development. Ann N Y Acad Sci. 1990. 593:259–271.11. Nilsen-Hamilton M. Transforming growth factor-beta and its actions on cellular growth and differentiation. Curr Top Dev Biol. 1990. 24:95–136.12. Millan FA, Denhez F, Konaiah P, Akhurst RJ. Embryonic gene expression patterns of TGFβ1, β2 and β3 suggest different development functions in vivo. Development (Camb.). 1991. 111:131–143.13. Pelton RW, Dickinson ME, Moses HL, Hogan BL. In situ hybridization analysis of TGFβ3 RNA expression during mouse development: comparative studies with TGF β1 and β2. Development (Camb.). 1990. 110:609–620.14. Schmid P, Cox D, Bilbe G, Maier R, McMaster GK. Differential expression of TGFβ1, β2 and β3 genes during mouse embryogenesis. Development (Camb.). 1991. 111:117–130.15. Pelton RW, Saxena B, Jones M, Moses HL, Gold LI. Immunohistochemical localization of TGFβ1, TGFβ2 and TGFβ3 in the mouse embryo: Expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991. 4:1091–1105.16. Takemura T, Sakagami M, Takebayashi K, Umemoto M, Nakase T, Takaoka K, Kubo T, Kitamura Y, Nomura S. Localization of bone morphogenetic protein-4 messenger RNA in developing mouse cochlea. Hear Res. 1996. 95:26–32.
Article17. Paradies NE, Sanford LP, Doetschman T, Friedman RA. Developmental expression of the TGFβ in the mouse cochlea. Mech Dev. 1998. 79:165–168.18. Wu DK, Oh SH. Sensory organ generation in the chick inner ear. J Neurosci. 1996. 16:6454–6462.
Article19. Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998. 18:3327–3335.
Article20. Cole LK, Le Roux I, Nunes F, Laufer E, Lewis J, Wu DK. Sensory organ generation in the chicken inner ear: contributions of bone morphogenetic protein 4, serrate1, and lunatic fringe. J Comp Neurol. 2000. 424:509–520.21. Kil SH, Collazo A. Origin of inner ear sensory organs revealed by fate map and time lapse analyses. Dev Biol. 2001. 233:365–379.22. Frenz DA, Van de Water TR, Galinovic-Schwartz V. Transforming growth factor beta: does it direct otic capsule formation? Ann Otol Rhinol Laryngol. 1991. 100:301–307.
Article23. Frenz DA, Galinovic-Schwartz V, Liu W, Flanders KC, Van de Water TR. Transforming growth factor beta 1 is an epithelial-derived signal peptide that influence otic capsule formation. Dev Biol. 1992. 153:324–336.24. Frenz DA, Liu W. Treatment with all-trans-retinoic acid decreases levels of endogenous TGF-beta (1) in the mesenchyme of the developing mouse inner ear. Teratology. 2000. 61:297–304.25. Butts SC, Liu W, Li G, Frenz DA. Transforming growth factor-β1 signaling participates in the physiological and pathological regulation of mouse inner ear development by all-trans retinoic acid. Birth Defects Res A Clin Mol Teratol. 2005. 73:218–228.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of alpha-Lipoic Acid on Proteinuria and Renal TGFbeta Expression in Obese Type 2 Diabetic Rat Model
- Effect of Retinoic Acid on Palate Formation during Rat Embryogenesis
- Over-expression of EphrinA2 in the Anterior Region of the Developing Mouse Midbrain and Diencephalon
- Study on the Expression of Insulin-like Growth Factor II (IGF- II) in Hepatocellular Carcinoma Cells and Developing Rat Embryos
- Expression of AQP-4 in Developing Rat Stomach: An Immunohistochemical Study