J Korean Med Sci.
2006 Feb;21(1):126-130. 10.3346/jkms.2006.21.1.126.
Prognostic Implication of Telomerase Activity in Patients with Brain Tumors
- Affiliations
-
- 1Department of Neurosurgery, Hanyang University College of Medicine, Seoul, Korea. kch5142@hanyang.ac.kr
- KMID: 2157795
- DOI: http://doi.org/10.3346/jkms.2006.21.1.126
Abstract
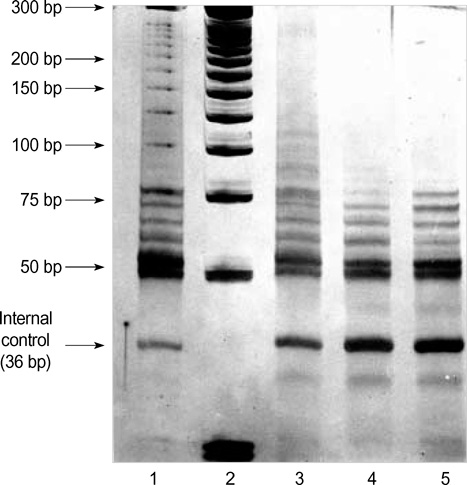
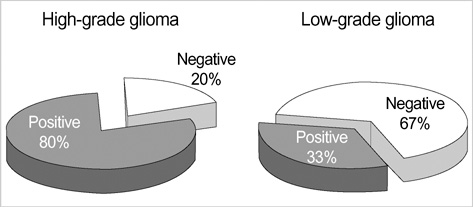
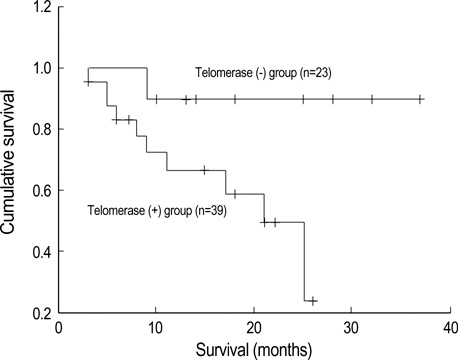
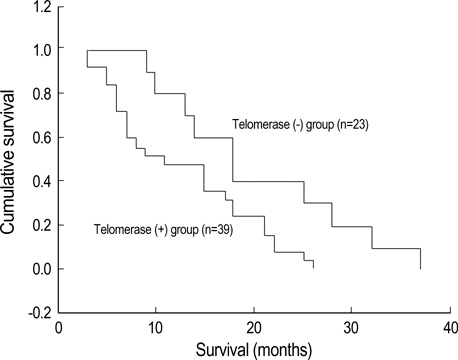
- Telomerase adds telomeric repeats to the ends of telomeres to compensate for their progressive loss. A favorable prognosis is associated with low or no telomerase in some tumors. The authors investigated whether telomerase activity is associated with survival of patients with brain tumors. Sixty-two consecutive patients with brain tumors underwent surgery, and their surgical specimens were investigated. The patients were pathologically categorized as group I (aggressive group) and group II (non-aggressive group). Telomerase activity was examined by the telomeric repeat amplification protocol (TRAP) assay. The median time was calculated in association with overall survival and progression-free survival in each group. The significant difference was noted in telomerase activity between high-grade gliomas and lowgrade gliomas (p=0.022). Telomerase activity was significantly associated with the median overall survival and progression-free survival in all tumors of the aggressive group. On the other hand, the median overall survival in the non-aggressive group was not dependent on telomerase activity, while the median progression-free survival was. Our data suggests that telomerase is an important prognostic indicator of survival in patients with brain tumors.
Keyword
MeSH Terms
Figure
Reference
-
1. Blackburn EH. Structure and function of telomeres. Nature. 1991. 350:569–573.
Article2. de Lange T. Activation of telomerase in a human tumor. Proc Natl Acad Sci USA. 1994. 91:2882–2885.
Article3. Greider CW. Telomerase activity, cell proliferation, and cancer. Proc Natl Acad Sci USA. 1998. 95:90–92.
Article4. Harley CB, Futcher AB, Geider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990. 345:458–460.
Article5. Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass MB, Arruda I, Robinson MO. A mammalian telomerase-associated protein. Science. 1997. 275:973–977.
Article6. Hanh WC. New functions for telomerase. Cancer Res Treat. 2003. 35:467–471.
Article7. Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985. 43:405–413.
Article8. Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrieh SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994. 266:2011–2015.
Article9. Falchetti ML, Larocca LM, Pallini R. Telomerase in brain tumors. Childs Nerv Syst. 2002. 18:112–117.
Article10. Hiraga S, Ohnishi T, Izumoto S, Miyahara E, Kanemura Y, Matsumura H, Arita N. Telomerase activity and alterations in telomere length in human brain tumors. Cancer Res. 1998. 58:2117–2125.11. Yoshino A, Katayama Y, Fukushima T, Watanabe T, Komine C, Yokoyama T, Kusama K, Moro I. Telomerase activity in pituitary adenomas: significance of telomerase expression in predicting pituitary adenoma recurrence. J Neurooncol. 2003. 63:155–162.12. Holt SE, Glinsky VV, Ivanova AB, Glinsky GV. Resistance to apoptosis in human cells conferred by telomerase function and telomere stability. Mol Carcinog. 1999. 25:241–248.
Article13. Kepes JJ. Review of the WHO's proposed new classification of brain tumors. Proceedings of the XIth International Congress of Neuropathology. September 2-8, 1990; Kyoto. Kyoto, Japan: Japanese Society of Neuropathology.14. Harrington L, Zhou W, McPhail T, Oulton R, Yeung DS, Mar V, Bass MB, Robinson MO. Human telomerase contains evolutionarily conserved catalytic and structural subunits. Genes Dev. 1997. 11:3109–3115.15. Harada K, Kurisu K, Arita K, Sadatomo T, Tahara H, Tahara E, Ide T, Uozumi T. Telomerase activity in central nervous system malignant lymphoma. Cancer. 1999. 86:1050–1055.
Article16. Park WC, Jung SS, Kim IC. Diagnostic significance of telomerase activity in breast cancer. J Korean Surg Soc. 2000. 58:767–774.17. Lee HJ, Myung SJ, Park YH, Cho YK, Jung HY, Lee GH, Hong WS, Yang SK, Kim JH, Min YI. Measurement of telomerase activity and telomerase reverse transcriptase expression in gastric fluid and tissue for early diagnosis of stomach cancer. Korean J Gatroenterol. 2003. 42:183–189.18. Wu TC, Lin P, Hsu CP, Huang YJ, Chen CY, Chung WC, Lee H, Ko JL. Loss of telomerase activity may be a potential favorable prognostic marker in lung carcinoma. Lung Cancer. 2003. 41:163–169.19. Okayasu I, Mitomi H, Yamashita K, Mikami T, Fujiwara M, Kato M, Oshimura M. Telomerase activity significantly correlates with cell differentiation, proliferation and lymph node metastasis in colorectal carcinoma. J Cancer Res Clin Oncol. 1998. 124:444–449.20. Poremba C, Hero B, Heine B, Scheel C, Schaefer KL, Christiansen H, Berthold F, Kneif S, Stein H, Juergens H, Boecker W, Dockhorn-Dworniczak B. Telomerase is a strong indicator for assessing the proneness to progression in neuroblastomas. Med Pediatr Oncol. 2000. 35:651–655.
Article21. Kudoh C, Sugiura K, Hisatomi H, Detta A, Yeh J. Detection of telomerase RNA component and telomerase activity in human brain tumors. 49th Annual Meeting of Congress of Neurological Surgeons. 1999. Boston, USA: –Abstract p645.22. Kleinschmidt-DeMasters BK, Shroyer AL, Hashizumi TL, Evans LC, Markham N, Kindt G, Shroyer KR. Part I. Telomerase levels in human metastatic brain tumors four-fold logarithmic variability but no correlation with tumor type or interval to patient demise. J Neurol Sci. 1998. 161:116–123.23. Liao CT, Tung-Chieh Chang J, Wang HM, Chen IH, Lin CY, Chen TM, Hsieh LL, Cheng AJ. Telomerase as an independent prognostic factor in head and neck squamous cell carcinoma. Head Neck. 2004. 26:504–512.
Article24. Koscielny S, Dahse R, Sonntag J, Riese U, Theuer C, Hoffman ME, von Eggeling F, Claussen U, Beleites E, Ernst G, Fiedler W. Clinical implications of telomerase activity and inactivation of the tumor suppressor gene p16 (CDKN2A) in head and neck cancer. Otolaryngol Pol. 2000. 54:291–295.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Telomerase Activity and Correlation with Histological Malignancy and Prognosis in Brain Tumors
- Effect of Bcl-2 Expression and Telomerase Activity on Apoptosis of the Brain Tumors
- Expression of Telomerase Activity and Apoptosis in Human Brain Tumors
- Telomerase Activity in Musculoskeletal Tumors
- Telomerase Activity and Expression of Telomerase RNA in Malignant Fibrous Histiocytoma