J Korean Med Sci.
2006 Feb;21(1):30-34. 10.3346/jkms.2006.21.1.30.
Impact of Circulating TGF-beta and IL-10 on T Cell Cytokines in Patients with Asthma and Tuberculosis
- Affiliations
-
- 1Asthma and Allergy Research Group, Allergy and Respiratory Diseases, Soonchunhyang University College of Medicine, Seoul, Korea. mdcspark@unitel.co.kr
- KMID: 2157778
- DOI: http://doi.org/10.3346/jkms.2006.21.1.30
Abstract
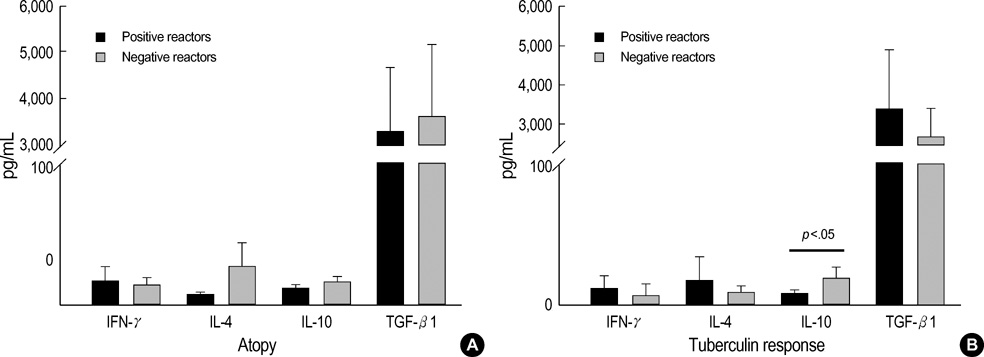
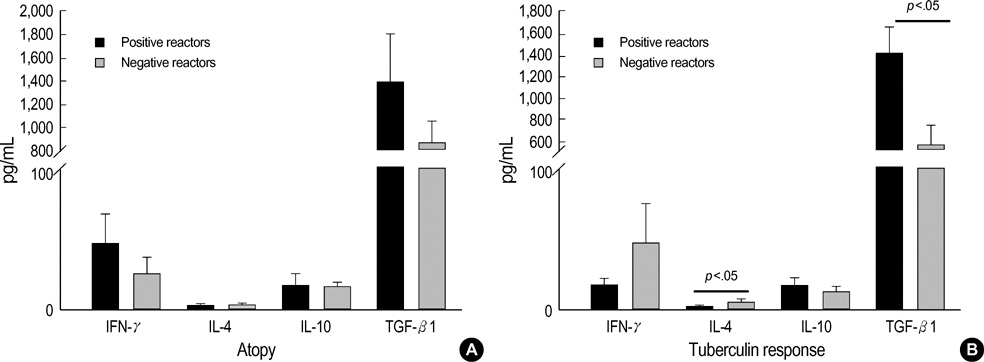
- Regulatory T cells, which stimulate or inhibit the effector functions of distinct T cell subsets, are critical in the control of the immune response. We investigated the effect of TGF-beta and IL-10 on T cell subsets according to the Th1/Th2 immune status. Sixty-two patients with asthma and 38 patients with pulmonary tuberculosis were included. Allergy skin tests, tuberculin tests, and chest radiography were performed. The levels of circulating IL-4, IFN-gamma, TGF-beta1, and IL-10 were measured using ELISA. The level of TGF-beta1 was higher in patients with asthma than in those with tuberculosis, but the IL-10 levels were the same between the asthma and tuberculosis groups. Atopy was unrelated to the tuberculin response. The IFN-gamma level was correlated with the IL-10 level, and the level of IL-4 was unrelated to the IL-10 or TGF-beta1 level. The level of IL-10 was higher in the negative tuberculin reactors than in the positive tuberculin reactors among patients with asthma, and TGF-beta1 was higher in the positive tuberculin reactors than in the negative tuberculin reactors among patients with tuberculosis. These results demonstrate that the regulatory effects of circulating TGF-beta and IL-10 on T cell cytokines may be different between Th2-type asthma and Th1 tuberculosis.
MeSH Terms
-
Adult
Asthma/*blood/immunology
Cytokines/*blood
Female
Humans
Interferon Type II/blood
Interleukin-10/blood
Interleukin-4/blood
Male
Research Support, Non-U.S. Gov't
Respiratory Function Tests
Skin Tests
Th1 Cells/*metabolism
Th2 Cells/*metabolism
Transforming Growth Factor beta/blood
Tuberculin Test
Tuberculosis/*blood/immunology
Figure
Reference
-
1. National Asthma Education and Prevention Program Expert Panel Report 2: Guidelines for the diagnosis and management of asthma. 1997. Bethesda (MD): National Institutes of Health, National Heart, Lung, and Blood Institute;publication No: 97-4051A.2. Chan J, Kaufman SH. Bloom BR, editor. Immune mechanisms of protection. Tuberculosis: Pathogenesis, protection and control. 1994. Washington: American society for microbiology press;389–415.
Article3. Shaheen SO. Changing patterns of childhood infection and the rise in allergic disease. Clin Exp Allergy. 1995. 25:1034–1037.4. Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997. 275:77–79.
Article5. Akbari O, Stock P, DeKruyff RH, Umetsu DT. Role of regulatory T cells in allergy and asthma. Curr Opin Immunol. 2003. 15:627–633.
Article6. Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994. 265:1237–1240.
Article7. American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991. 144:1202–1218.8. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease and asthma. Am Rev Respir Dis. 1987. 136:225–244.9. Park CS, Kim YY, Kang SY. Collection between RAST and skin test for inhalant offending allergens. J Korean Soc Allergol. 1983. 3:1–9.10. Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998. 187:875–883.
Article11. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone, I: definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986. 136:2348–2357.12. Del Prete GF, De Carli M, Mastromauro C, Bigiotti R, Macchia D, Falagiani P, Ricci M, Romagnani S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen (s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991. 88:346–350.13. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The group health medical associates. N Engl J Med. 1995. 332:133–138.14. Holt PG, Sly PD, Bjorksten B. Atopic versus infectious diseases in childhood: a question of balance? Pediatr Allergy Immunol. 1997. 8:53–58.
Article15. Lacour M. Acute infections in atopic dermatitis: a clue for a pathogenic role of a Th1/Th2 imbalance? Dermatology. 1994. 188:255–257.
Article16. Shaheen SO, Aaby P, Hall AJ, Barker DJ, Heyes CB, Shiell AW, Goudiaby A. Cell mediated immunity after measles in Guinea-Bissau: historical cohort study. Br Med J. 1996. 313:969–974.
Article17. Herz U, Gerhold K, Grüber C, Braun A, Wahn U, Renz H, Paul K. BCG infection suppresses allergic sensitization and development of increased airway reactivity in an animal model. J Allergy Clin Immunol. 1998. 102:867–874.
Article18. Mills KH, Barnard A, Watkins J, Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993. 61:399–410.
Article19. Yeung VP, Gieni RS, Umetsu DT, Dekruyff RH. Heat-killed Listeria monocytogenes as an adjuvant converts established murine Th2-dominated immune responses into Th1-dominated responses. J Immunol. 1998. 161:4146–4152.20. Scharton TM, Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993. 178:567–577.
Article21. Schlaak JF, Nieder P, Meyer zum Buschenfelde KH, Fleisher B. Human T helper cells reactive with somatic bacterial antigens belong to the Th1 subset. Med Microbiol Immunol Berl. 1994. 183:169–175.
Article22. Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002. 8:1024–1032.
Article23. Letterio JJ, Roberts AB. Regulation of immune responses by TGF-β. Annu Rev Immunol. 1998. 16:137–161.
Article24. Gorelik L, Flavell RA. Abrogation of TGF-β signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000. 12:171–181.
Article25. Lucas PJ, Kim SJ, Melby SJ, Gress RE. Disruption of T cell homeostasis in mice expressing a T cell-specific dominant negative transforming growth factor-β II receptor. J Exp Med. 2000. 191:1187–1196.26. Nakao A, Miike S, Hatano M, Okumura K, Tokuhisa T, Ra C, Iwamoto I. Blockade of transforming growth factor β/Smad signaling in T cells by overexpression of Smad7 enhances antigen-induced airway inflammation and airway reactivity. J Exp Med. 2000. 192:151–158.
Article27. Schramm C, Herz U, Podlech J, Protschka M, Finotto S, Reddehase MJ, Kohler H, Galle PR, Lohse AW, Blessing M. TGF-βII regulates airway responses via T cells. J Immunol. 2003. 170:1313–1319.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Circulating Cytokine Levels and Changes During the Treatment in Patients with Active Tuberculosis in Korea
- Effects of AII Receptor Blocker on the Urinary Excretion of IL-6 and TGF-beta in IgA Nephropathy
- Regulation of TNF - alpha Gene Expression in Human Fetal Astrocytes
- Effects of TGF-beta, GM-CSF, and PDGF on Proliferation and Expression of Cytokine and Metalloproteinase Genes in Rheumatoid Synovial Cells
- The Production and Correlation of Silica Induced Proinflammatory Cytokines and TGF-beta from Monocytes of Balb/C Mice