Ann Dermatol.
2009 Nov;21(4):409-412. 10.5021/ad.2009.21.4.409.
Coexistence of Amelanotic Melanoma and Liposarcoma
- Affiliations
-
- 1Department of Dermatology, School of Medicine, Kyung Hee University, Seoul, Korea. crhaw@khmc.or.kr
- KMID: 2156508
- DOI: http://doi.org/10.5021/ad.2009.21.4.409
Abstract
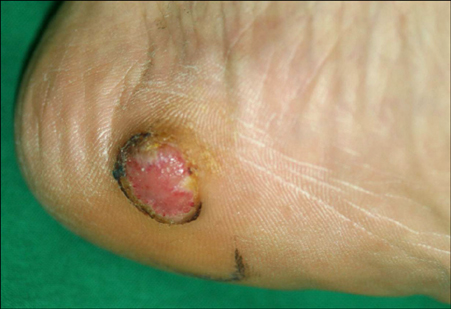
- An amelanotic malignant melanoma is characterized by little or no pigment. It is frequently misdiagnosed because it is a rare entity in general, and because of its unusual clinical features. Liposarcoma is one of the most common adult soft tissue sarcomas. We encountered a case of amelanotic melanoma with a concurrent liposarcoma. A 68-year-old man presented with a single, 1.5x1.5 cm round erythematous, eroded nodule on the left heel. A biopsy specimen showed atypical, pleomorphic tumor cells with little melanin pigment. The tumor cells were positive for S-100, HMB-45 and negative for cytokeratins. These findings were consistent with amelanotic melanoma. On positron emission tomography/computed tomography (PET/CT), a hypermetabolic lesion was found in the left buttock. This lesion was excised and diagnosed as a well-differentiated liposarcoma. An association between sarcomas and other primary malignancies has been reported. However, an association between melanoma and liposarcoma is rare.
Keyword
MeSH Terms
Figure
Reference
-
1. Pizzichetta MA, Talamini R, Stanganelli I, Puddu P, Bono R, Argenziano G, et al. Amelanotic/hypomelanotic melanoma: clinical and dermoscopic features. Br J Dermatol. 2004. 150:1117–1124.
Article2. Kim JG, Chin HS, Cho CK. A case report of amelanotic melanoma. Korean J Dermatol. 1977. 15:511–515.3. Kang YS, Park CW, Lee CH. A case of amelanotic melanoma. Ann Dermatol. 1994. 6:179–182.
Article4. Park HJ, Lee DW, Choi SW, Cho BK, Cho MJ, Shim SI. A case of amelanotic melanoma developing in a burn scar. Korean J Dermatol. 1995. 33:339–343.5. Kim SJ, Park HJ, Lee JY, Cho BK. A case of amelanotic subungual melanoma. Ann Dermatol. 2008. 20:26–28.
Article6. Ji J, Hemminki K. Second primary malignancies among patients with soft tissue tumors in Sweden. Int J Cancer. 2006. 119:909–914.
Article7. Brady MS, Brennan MF. Allen-Mersh TG, editor. Soft tissue sarcoma. Surgical oncology. 1996. London: Chapman and Hall;401–420.8. Fletcher CDM, Unni KK, Mertens F. World Health Organization classification of tumours. Pathology and genetics of tumours of soft tissue and bone. 2002. Lyon: IARC Press;40–44. 227–232.9. Berking C, Brady MS. Cutaneous melanoma in patients with sarcoma. Cancer. 1997. 79:843–848.
Article10. Merimsky O, Kollender Y, Issakov J, Bickels J, Flusser G, Gutman M, et al. Multiple primary malignancies in association with soft tissue sarcomas. Cancer. 2001. 91:1363–1371.
Article11. Dunbar SF, Marks LB, Sober AJ, Rosenberg A, Suit HD. Connective tissue tumors in patients with cutaneous melanoma. J Am Acad Dermatol. 1994. 31:216–219.
Article12. Boice JD Jr, Storm HH, Curtis RE, Jensen OM, Kleinerman RA, Jensen HS, et al. Introduction to the study of multiple primary cancers. Natl Cancer Inst Monogr. 1985. 68:3–9.13. Sharpless E, Chin L. The INK4a/ARF locus and melanoma. Oncogene. 2003. 22:3092–3098.
Article14. Bishop DT, Demenais F, Goldstein AM, Bergman W, Bishop JN, Bressac-de Paillerets B, et al. Geographical variation in the penetrance of CDKN2A mutations for melanoma. J Natl Cancer Inst. 2002. 94:894–903.
Article15. Wolfel T, Hauer M, Schneider J, Serrano M, Wolfel C, Klehmann-Hieb E, et al. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 1995. 269:1281–1284.
Article16. Pilotti S, Della Torre G, Lavarino C, Sozzi G, Minoletti F, Vergani B, et al. Molecular abnormalities in liposarcoma: role of MDM2 and CDK4-containing amplicons at 12q13-22. J Pathol. 1998. 185:188–190.
Article17. Hostein I, Pelmus M, Aurias A, Pedeutour F, Mathoulin-Pelissier S, Coindre JM. Evaluation of MDM2 and CDK4 amplification by real-time PCR on paraffin wax-embedded material: a potential tool for the diagnosis of atypical lipomatous tumours/well-differentiated liposarcomas. J Pathol. 2004. 202:95–102.
Article18. Shin S, Palis BE, Phillips JL, Stewart AK, Perry RR. Commission on Cancer Melanoma Disease Site Team. Cutaneous melanoma in Asian-Americans. J Surg Oncol. 2009. 99:114–118.
Article