Ann Dermatol.
2008 Dec;20(4):260-262. 10.5021/ad.2008.20.4.260.
Toxic Epidermal Necrolysis Induced by the Topical Carbonic Anhydrase Inhibitors Brinzolamide and Dorzolamide
- Affiliations
-
- 1Department of Dermatology, Chonnam National University Medical School, Gwangju, Korea. schul@chonnam.ac.kr
- KMID: 2156410
- DOI: http://doi.org/10.5021/ad.2008.20.4.260
Abstract
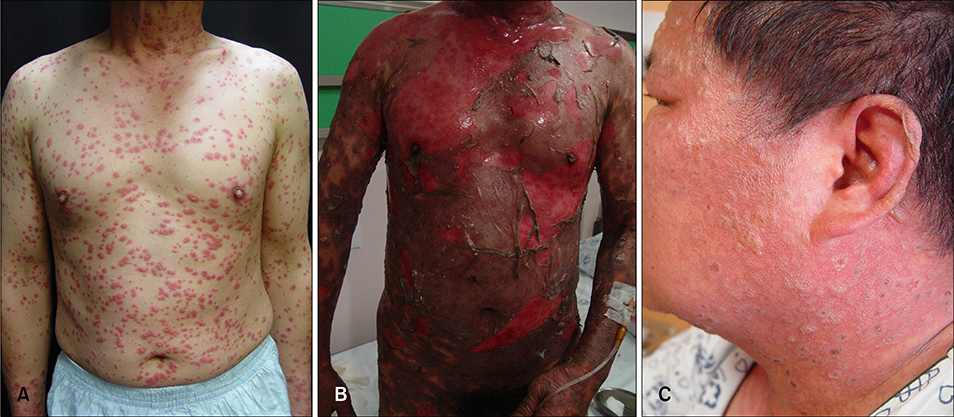
- Brinzolamide and dorzolamide are highly specific topical carbonic anhydrase inhibitors (CAIs). They lower intraocular pressure (IOP) by reducing the rate of aqueous humour formation without serious side effects. Although systemic CAIs are the most potent medications for lowering intraocular pressure for conditions with ocular hypertension, many cases with adverse systemic reactions have been reported, including Stevens-Johnson syndrome (SJS) and Toxic epidermal necrolysis (TEN). Here, we report 2 cases of TEN that were associated with topical CAIs rather than systemic CAIs.
MeSH Terms
Figure
Cited by 1 articles
-
Toxic Epidermal Necrolysis in a Patient with HLA-B*5901 Haplotype Caused by Topical and Oral Carbonic Anhydrase Inhibitors
Minseok Cheon, Young Bok Lee, Dong-Soo Yu, Jin-Wou Kim
Ann Dermatol. 2014;26(5):645-646. doi: 10.5021/ad.2014.26.5.645.
Reference
-
1. Cvetkovic RS, Perry CM. Brinzolamide: a review of its use in the management of primary open-angle glaucoma and ocular hypertension. Drugs Aging. 2003; 20:919–947.2. Herkel U, Pfeiffer N. Update on topical carbonic anhydrase inhibitors. Curr Opin Ophthalmol. 2001; 12:88–93.
Article3. Sulfonamide cross reactions explained. SDIS Drug News. 2003; 20(2):April-May.4. DeSantis L. Preclinical overview of brinzolamide. Surv Ophthalmol. 2000; 44:S119–S129.5. Mincione F, Scozzafava A, Supuran CT. The development of topically acting carbonic anhydrase inhibitors as anti-glaucoma agents. Curr Top Med Chem. 2007; 7:849–854.
Article6. Alcon (USA) Inc. Azopt® (brinzolamide ophthalmic suspension) 1%. Prescribing information [Internet]. http://ecatalog.alcon.com/pi/Azopt_us_en.pdf.7. Alcon Laboratories (UK) Ltd. Azopt (brinzolamide eye drops suspension) 1%. Summary of product characteristics. eMC [Internet]. http://emc.medicines.org.uk/emc/assets/c/html/displaydoc.asp?documentid=2808.8. Florez A, Roson E, Conde A, Gonzalez B, GarciaDoval I, de la Torre C, et al. Toxic epidermal necrolysis secondary to timolol, dorzolamide, and latanoprost eyedrops. J Am Acad Dermatol . 2005; 53:909–911.
Article9. Radimer GF, Davis JH, Ackerman AB. Fumigantinduced toxic epidermal necrolysis. Arch Dermatol. 1974; 110:103–104.
Article10. Thompson JA Jr, Wansker BA. A case of contact dermatitis, erythema multiforme, and toxic epidermal necrolysis. J Am Acad Dermatol. 1981; 5:666–669.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Topical Carbonic Anhydrase Inhibitors on Nitric Oxide Production in Trabecular Meshwork Cells
- Toxic Epidermal Necrolysis and Stevens-Johnson Syndrome Caused by Topical Ophthalmic Use of Dorzolamide
- Toxic Epidermal Necrolysis in a Patient with HLA-B*5901 Haplotype Caused by Topical and Oral Carbonic Anhydrase Inhibitors
- The Changes in Intraocular Pressure Following the Combined Treatment of Systemic Acetazolamide and Topical Dorzolamide
- Two Cases of HLA-B59(+) Stevens-Johnson Syndrome (SJS)-Toxic Epidermal Necrolysis (TEN) Associated with Methazolamide Treatment