Korean J Radiol.
2015 Aug;16(4):776-782. 10.3348/kjr.2015.16.4.776.
Combined Radiofrequency Ablation and Double Anti-Angiogenic Protein Therapy to Increase Coagulation Efficacy: An Experimental Study in a Murine Renal Carcinoma Model
- Affiliations
-
- 1Imaging Science Research Center, Wonkwang University School of Medicine, Iksan 570-711, Korea. khy1646@wonkwang.ac.kr
- 2Department of Surgery, Wonkwang University School of Medicine, Iksan 570-711, Korea.
- 3Department of Radiology, Wonkwang University School of Medicine, Iksan 570-711, Korea.
- KMID: 2155550
- DOI: http://doi.org/10.3348/kjr.2015.16.4.776
Abstract
OBJECTIVE
To evaluate whether suppression of tumor microvasculature by double anti-angiogenic protein (DAAP) treatment could increase the extent of radiofrequency ablation (RFA)-induced coagulation in a murine renal cell carcinoma model.
MATERIALS AND METHODS
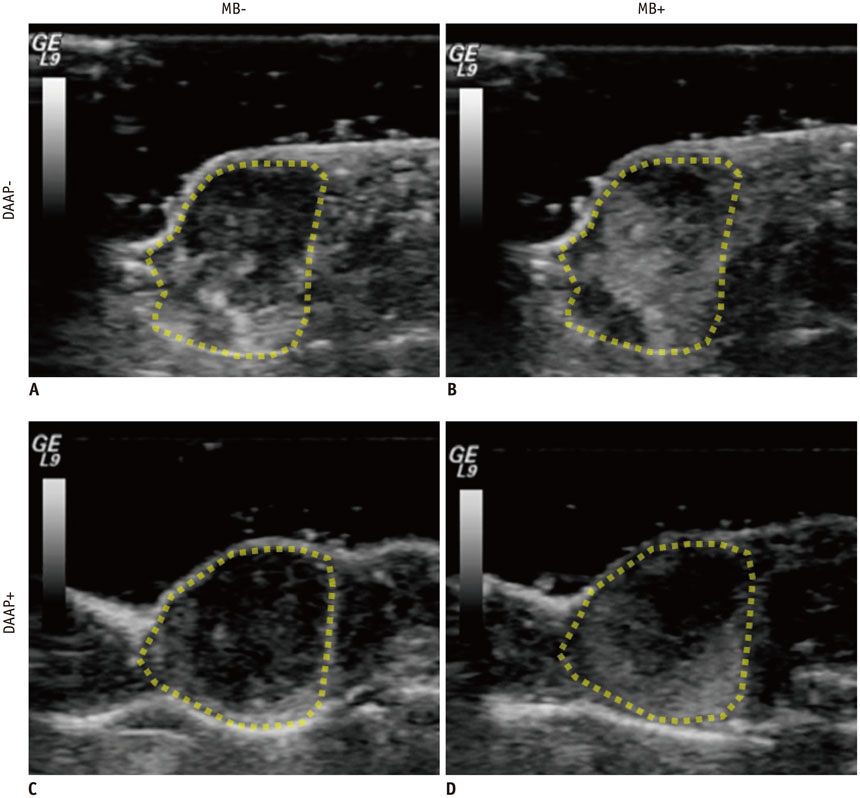
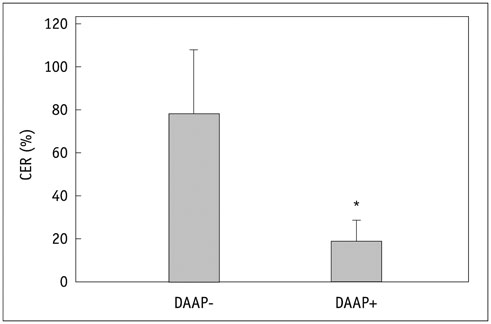
Renal cell carcinoma cell lines were implanted subcutaneously into 10 nude mice. Four mice received adenoviral DAAP treatment and 6 mice received sterile 0.9% saline solution as DAAP-untreated group. The effect of DAAP was evaluated according to the vascularity by contrast-enhanced ultrasound (CEUS) using microbubbles. Four DAAP-treated mice and 4 DAAP-untreated mice were then treated with RFA, resulting in 3 groups: no-therapy (n = 2), RFA only (n = 4), and RFA combined with DAAP treatment (n = 4). Immediately after RFA, the size of coagulation necrosis and mitochondrial enzyme activity were compared between the groups using analysis of variance (ANOVA) and post hoc test.
RESULTS
The contrast enhancement ratio for tumor vascularization on CEUS was significantly lower in the DAAP treated group than in DAAP-untreated group (30.2 +/- 9.9% vs. 77.4 +/- 17.3%; p = 0.021). After RFA, the mean coagulation diameter was 0 mm for no-therapy group, 6.7 +/- 0.7 mm for the RFA only group and 8.5 +/- 0.4 mm for the RFA with DAAP group (ANOVA, p < 0.001). The area of viable mitochondria within the tumor was 27.9 +/- 3.9% in no-therapy group, 10.3 +/- 4.5% in the RFA only group, and 2.1 +/- 0.7% in the RFA with DAAP group (ANOVA, p < 0.001).
CONCLUSION
Our results suggest the potential value of combining RFA with anti-angiogenic therapy.
Keyword
MeSH Terms
-
Adenoviridae
Angiogenic Proteins/*antagonists & inhibitors
Animals
Carcinoma, Renal Cell/blood supply/surgery/*therapy
Catheter Ablation/*methods
Combined Modality Therapy
Contrast Media
Kidney Neoplasms/blood supply/surgery/*therapy
Male
Mice
Mice, Nude
Microbubbles
Neovascularization, Pathologic/surgery/*therapy/ultrasonography
Recombinant Proteins
Angiogenic Proteins
Contrast Media
Recombinant Proteins
Figure
Cited by 1 articles
-
Percutaneous Dual-Switching Monopolar Radiofrequency Ablation Using a Separable Clustered Electrode: A Preliminary Study
Tae Won Choi, Jeong Min Lee, Dong Ho Lee, Jeong-Hoon Lee, Su Jong Yu, Yoon Jun Kim, Jung-Hwan Yoon, Joon Koo Han
Korean J Radiol. 2017;18(5):799-808. doi: 10.3348/kjr.2017.18.5.799.
Reference
-
1. Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010; 46:765–781.2. Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010; 58:398–406.3. Kim SY, Woo S, Hwang SI, Moon MH, Sung CK, Lee HJ, et al. Usefulness of resistive index on spectral Doppler ultrasonography in the detection of renal cell carcinoma in patients with end-stage renal disease. Ultrasonography. 2014; 33:136–142.4. Tranquart F, Correas JM, Martegani A, Greppi B, Bokor D. [Feasability of real time contrast enhanced ultrasound in renal disease]. J Radiol. 2004; 85:31–36.5. Choueiri TK, Schutz FA, Hevelone ND, Nguyen PL, Lipsitz SR, Williams SB, et al. Thermal ablation vs surgery for localized kidney cancer: a Surveillance, Epidemiology, and End Results (SEER) database analysis. Urology. 2011; 78:93–98.6. Takaki H, Yamakado K, Soga N, Arima K, Nakatsuka A, Kashima M, et al. Midterm results of radiofrequency ablation versus nephrectomy for T1a renal cell carcinoma. Jpn J Radiol. 2010; 28:460–468.7. Gervais DA. Cryoablation versus radiofrequency ablation for renal tumor ablation: time to reassess? J Vasc Interv Radiol. 2013; 24:1135–1138.8. Raman JD, Hall DW, Cadeddu JA. Renal ablative therapy: radiofrequency ablation and cryoablation. J Surg Oncol. 2009; 100:639–644.9. Pirasteh A, Snyder L, Boncher N, Passalacqua M, Rosenblum D, Prologo JD. Cryoablation vs. radiofrequency ablation for small renal masses. Acad Radiol. 2011; 18:97–100.10. Park S, Anderson JK, Matsumoto ED, Lotan Y, Josephs S, Cadeddu JA. Radiofrequency ablation of renal tumors: intermediate-term results. J Endourol. 2006; 20:569–573.11. Stern JM, Svatek R, Park S, Hermann M, Lotan Y, Sagalowsky AI, et al. Intermediate comparison of partial nephrectomy and radiofrequency ablation for clinical T1a renal tumours. BJU Int. 2007; 100:287–290.12. Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow. Comparison with standard percutaneous radiofrequency ablation therapy. Cancer. 2002; 95:2353–2360.13. Shen P, Fleming S, Westcott C, Challa V. Laparoscopic radiofrequency ablation of the liver in proximity to major vasculature: effect of the Pringle maneuver. J Surg Oncol. 2003; 83:36–41.14. Koh YJ, Kim HZ, Hwang SI, Lee JE, Oh N, Jung K, et al. Double antiangiogenic protein, DAAP, targeting VEGF-A and angiopoietins in tumor angiogenesis, metastasis, and vascular leakage. Cancer Cell. 2010; 18:171–184.15. Jung K, Lee D, Lim HS, Lee SI, Kim YJ, Lee GM, et al. Double anti-angiogenic and anti-inflammatory protein Valpha targeting VEGF-A and TNF-alpha in retinopathy and psoriasis. J Biol Chem. 2011; 286:14410–14418.16. Ravaud A, Gross-Goupil M, Bellmunt J. Combination therapy in metastatic renal cell cancer. Semin Oncol. 2013; 40:472–481.17. Jun HY, Park SH, Kim HS, Yoon KH. Long residence time of ultrasound microbubbles targeted to integrin in murine tumor model. Acad Radiol. 2010; 17:54–60.18. Zhang Q, Yang H, Kang SJ, Wang Y, Wang GD, Coulthard T, et al. In vivo high-frequency, contrast-enhanced ultrasonography of uveal melanoma in mice: imaging features and histopathologic correlations. Invest Ophthalmol Vis Sci. 2011; 52:2662–2668.19. Hwang M, Hariri G, Lyshchik A, Hallahan DE, Fleischer AC. Correlation of quantified contrast-enhanced sonography with in vivo tumor response. J Ultrasound Med. 2010; 29:597–607.20. Shiyan L, Pintong H, Zongmin W, Fuguang H, Zhiqiang Z, Yan Y, et al. The relationship between enhanced intensity and microvessel density of gastric carcinoma using double contrast-enhanced ultrasonography. Ultrasound Med Biol. 2009; 35:1086–1091.21. Horkan C, Ahmed M, Liu Z, Gazelle GS, Solazzo SA, Kruskal JB, et al. Radiofrequency ablation: effect of pharmacologic modulation of hepatic and renal blood flow on coagulation diameter in a VX2 tumor model. J Vasc Interv Radiol. 2004; 15:269–274.22. Hines-Peralta A, Sukhatme V, Regan M, Signoretti S, Liu ZJ, Goldberg SN. Improved tumor destruction with arsenic trioxide and radiofrequency ablation in three animal models. Radiology. 2006; 240:82–89.23. Wang C, Yu C, Yang F, Yang G. Diagnostic accuracy of contrast-enhanced ultrasound for renal cell carcinoma: a meta-analysis. Tumour Biol. 2014; 35:6343–6350.24. Siracusano S, Quaia E, Bertolotto M, Ciciliato S, Tiberio A, Belgrano E. The application of ultrasound contrast agents in the characterization of renal tumors. World J Urol. 2004; 22:316–322.25. Williams R, Hudson JM, Lloyd BA, Sureshkumar AR, Lueck G, Milot L, et al. Dynamic microbubble contrast-enhanced US to measure tumor response to targeted therapy: a proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy. Radiology. 2011; 260:581–590.26. Solbiati L, Ierace T, Tonolini M, Cova L. Guidance and monitoring of radiofrequency liver tumor ablation with contrast-enhanced ultrasound. Eur J Radiol. 2004; 51:S19–S23.27. Liu JB, Goldberg BB, Merton DA, Rawool NM, Needleman L, Forsberg F. The role of contrast-enhanced sonography for radiofrequency ablation of liver tumors. J Ultrasound Med. 2001; 20:517–523.28. Hines-Peralta A, Goldberg SN. Review of radiofrequency ablation for renal cell carcinoma. Clin Cancer Res. 2004; 10(18 Pt 2):6328S–6334S.29. Hakimé A, Hines-Peralta A, Peddi H, Atkins MB, Sukhatme VP, Signoretti S, et al. Combination of radiofrequency ablation with antiangiogenic therapy for tumor ablation efficacy: study in mice. Radiology. 2007; 244:464–470.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current status and future of radiofrequency ablation for hepatocellular carcinoma
- The Role of Combination of Transarterial Chemoebolization and Radiofrequency Ablation for Hepatocellular Carcinoma Treatment
- Chemoembolization combined with radiofrequency ablation is the best option for the local treatment of early hepatocellular carcinoma?
- Spontaneous Regression of Pulmonary and Adrenal Metastases Following Percutaneous Radiofrequency Ablation of a Recurrent Renal Cell Carcinoma
- Radiofrequency Thermal Ablation of Hepatocellular Carcinomas