Cancer Res Treat.
2016 Jan;48(1):28-36. 10.4143/crt.2014.258.
Phase I Study of CKD-516, a Novel Vascular Disrupting Agent, in Patients with Advanced Solid Tumors
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. bangyj@snu.ac.kr
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- 4Department of Internal Medicine, Kangbuk Samsung Medical Center, Seoul, Korea.
- 5Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 6Department of Clinical Pharmacology and Therapeutics, Seoul National University Hospital, Seoul, Korea.
- KMID: 2152257
- DOI: http://doi.org/10.4143/crt.2014.258
Abstract
- PURPOSE
CKD-516 is a newly developed vascular disrupting agent. This phase I dose-escalation study of CKD-516 was conducted to determine maximum-tolerated dose (MTD), safety, pharmacokinetics, and preliminary antitumor efficacy in patients with advanced solid tumors.
MATERIALS AND METHODS
Patients received CKD-516 intravenously on D1 and D8 every 3 weeks, in a standard 3+3 design. Safety was evaluated by National Cancer Institute Common Terminology Criteria for Adverse Events ver. 4.02 and response was assessed by Response Evaluation Criteria in Solid Tumor ver. 1.1.
RESULTS
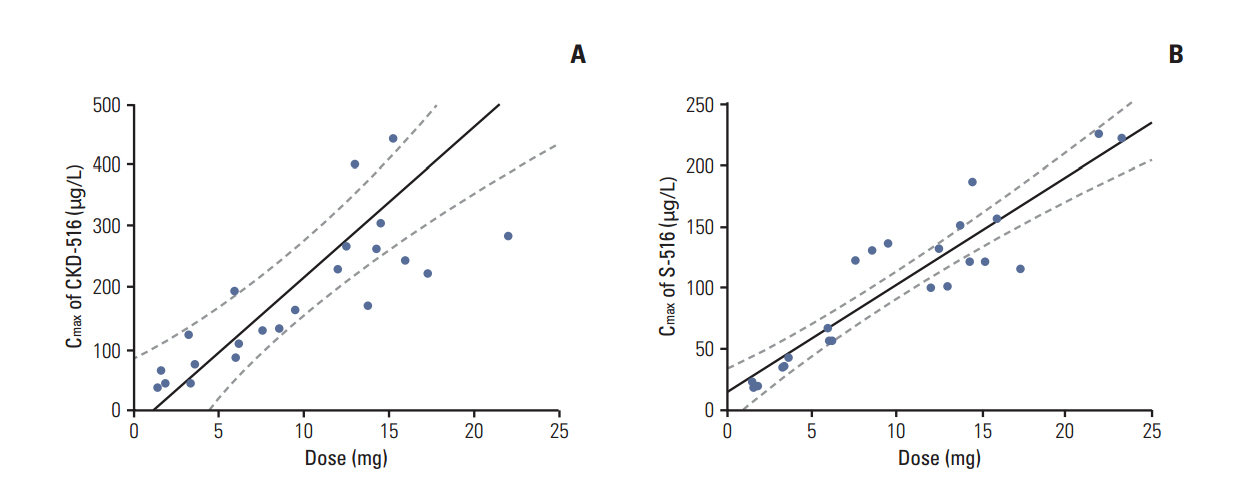
Twenty-three patients were treated with CKD-516 at seven dosing levels: 1 mg/m2/day (n=3), 2 mg/m2/day (n=3), 3.3 mg/m2/day (n=3), 5 mg/m2/day (n=3), 7 mg/m2/day (n=3), 9 mg/m2/day (n=6), and 12 mg/m2/day (n=2). Mean age was 54 and 56.5% of patients were male. Two dose-limiting toxicities, which were both grade 3 hypertension, were observed in two patients at 12 mg/m2/day. The MTD was determined as 12 mg/m2/day. Most common adverse events were gastrointestinal adverse events (diarrhea, 34.8% [30.4% grade 1/2, 13.0% grade 3]; nausea, 21.7% [all grade 1/2]; vomiting, 21.7% [all grade 1/2]), myalgia (17.4%, all grade 1/2), and abdominal pain (21.7% [21.7% grade 1/2, 4.3% grade 3]). The pharmacokinetic study showed the dose-linearity of all dosing levels. Among 23 patients, six patients (26.1%) showed stable disease. Median progression-free survival was 39 days (95% confidence interval, 37 to 41 days).
CONCLUSION
This study demonstrates feasibility of CKD-516, novel vascular disrupting agent, in patients with advanced solid tumor. MTD of CKD-516 was defined as 12 mg/m2/day on D1 and D8 every 3 weeks.
MeSH Terms
Figure
Reference
-
References
1. Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010; 9:790–803.
Article2. Siemann DW. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumorvascular disrupting agents. Cancer Treat Rev. 2011; 37:63–74.
Article3. Sessa C, Lorusso P, Tolcher A, Farace F, Lassau N, Delmonte A, et al. Phase I safety, pharmacokinetic and pharmacodynamic evaluation of the vascular disrupting agent ombrabulin (AVE8062) in patients with advanced solid tumors. Clin Cancer Res. 2013; 19:4832–42.
Article4. Eskens FA, Tresca P, Tosi D, Van Doorn L, Fontaine H, Van der Gaast A, et al. A phase I pharmacokinetic study of the vascular disrupting agent ombrabulin (AVE8062) and docetaxel in advanced solid tumours. Br J Cancer. 2014; 110:2170–7.
Article5. Li J, Jamin Y, Boult JK, Cummings C, Waterton JC, Ulloa J, et al. Tumour biomechanical response to the vascular disrupting agent ZD6126 in vivo assessed by magnetic resonance elastography. Br J Cancer. 2014; 110:1727–32.
Article6. Lee J, Kim SJ, Choi H, Kim YH, Lim IT, Yang HM, et al. Identification of CKD-516: a potent tubulin polymerization inhibitor with marked antitumor activity against murine and human solid tumors. J Med Chem. 2010; 53:6337–54.
Article7. Lee J, Bae S, Lee SH, Choi H, Kim YH, Kim SJ, et al. Discovery of a potent tubulin polymerization inhibitor: synthesis and evaluation of water-soluble prodrugs of benzophenone analog. Bioorg Med Chem Lett. 2010; 20:6327–30.
Article8. Moon CH, Lee SJ, Lee HY, Dung le TK, Cho WJ, Cha H, et al. CKD-516 displays vascular disrupting properties and enhances anti-tumor activity in combination with chemotherapy in a murine tumor model. Invest New Drugs. 2014; 32:400–11.
Article9. Kim YI, Kim KW, Lee HK, Park J, Chung JW, Youn H, et al. Enhanced efficacy of CKD-516 in combination with doxorubicin: pre-clinical evaluation using a hepatocellular carcinoma xenograft model. Anticancer Res. 2014; 34:1715–22.10. Rustin GJ, Galbraith SM, Anderson H, Stratford M, Folkes LK, Sena L, et al. Phase I clinical trial of weekly combretastatin A4 phosphate: clinical and pharmacokinetic results. J Clin Oncol. 2003; 21:2815–22.
Article11. Stevenson JP, Rosen M, Sun W, Gallagher M, Haller DG, Vaughn D, et al. Phase I trial of the antivascular agent combretastatin A4 phosphate on a 5-day schedule to patients with cancer: magnetic resonance imaging evidence for altered tumor blood flow. J Clin Oncol. 2003; 21:4428–38.
Article12. Dowlati A, Robertson K, Cooney M, Petros WP, Stratford M, Jesberger J, et al. A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin a-4 phosphate on a single-dose intravenous schedule in patients with advanced cancer. Cancer Res. 2002; 62:3408–16.13. Beerepoot LV, Radema SA, Witteveen EO, Thomas T, Wheeler C, Kempin S, et al. Phase I clinical evaluation of weekly administration of the novel vascular-targeting agent, ZD6126, in patients with solid tumors. J Clin Oncol. 2006; 24:1491–8.
Article14. Mita MM, Spear MA, Yee LK, Mita AC, Heath EI, Papadopoulos KP, et al. Phase 1 first-in-human trial of the vascular disrupting agent plinabulin(NPI-2358) in patients with solid tumors or lymphomas. Clin Cancer Res. 2010; 16:5892–9.15. Hande KR, Hagey A, Berlin J, Cai Y, Meek K, Kobayashi H, et al. The pharmacokinetics and safety of ABT-751, a novel, orally bioavailable sulfonamide antimitotic agent: results of a phase 1 study. Clin Cancer Res. 2006; 12:2834–40.
Article16. Joo I, Lee JM, Han JK, Choi BI. Intravoxel incoherent motion diffusion-weighted MR imaging for monitoring the therapeutic efficacy of the vascular disrupting agent CKD-516 in rabbit VX2 liver tumors. Radiology. 2014; 272:417–26.
Article17. Joo I, Kim JH, Lee JM, Choi JW, Han JK, Choi BI. Early quantification of the therapeutic efficacy of the vascular disrupting agent, CKD-516, using dynamic contrast-enhanced ultrasonography in rabbit VX2 liver tumors. Ultrasonography. 2014; 33:18–25.
Article18. Kim KW, Lee JM, Jeon YS, Kang SE, Baek JH, Han JK. Free-breathing dynamic contrast-enhanced MRI of the abdomen and chest using a radial gradient echo sequence with K-space weighted image contrast (KWIC). Eur Radiol. 2013; 23:1352–60.
Article19. Baguley BC. Preclinical efficacy of vascular disrupting agents in non-small-cell lung cancer. Clin Lung Cancer. 2011; 12:81–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Early quantification of the therapeutic efficacy of the vascular disrupting agent, CKD-516, using dynamic contrast-enhanced ultrasonography in rabbit VX2 liver tumors
- Phase 1/2a Study of Rivoceranib, a Selective VEGFR-2 Angiogenesis Inhibitor, in Patients with Advanced Solid Tumors
- Vascular Calcification in Chronic Kidney Disease: Distinct Features of Pathogenesis and Clinical Implication
- Immunotherapy in Pediatric Solid Tumors
- Personalized nutritional management in the transition from non-dialysis dependent chronic kidney disease to dialysis