J Pathol Transl Med.
2015 Jul;49(4):331-334. 10.4132/jptm.2015.04.16.
A Rare Case of Primary Tubular Adenocarcinoma of the Thymus, Enteric Immunophenotype: A Case Study and Review of the Literature
- Affiliations
-
- 1Department of Pathology, Soonchunhyang University Cheonan Hospital, Cheonan, Korea. mhoh0212@schmc.ac.kr
- 2Department of Pathology and Respiratory Center, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Division of Hematology and Oncology, Department of Internal Medicine, Soonchunhyang University Cheonan Hospital, Cheonan, Korea.
- KMID: 2151143
- DOI: http://doi.org/10.4132/jptm.2015.04.16
Abstract
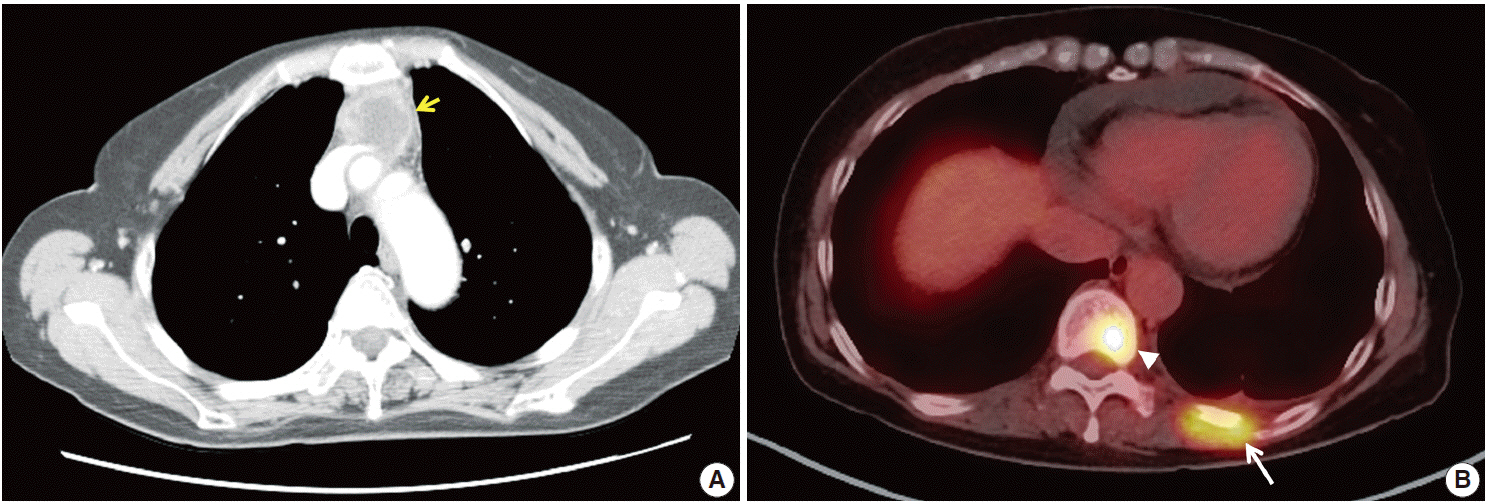
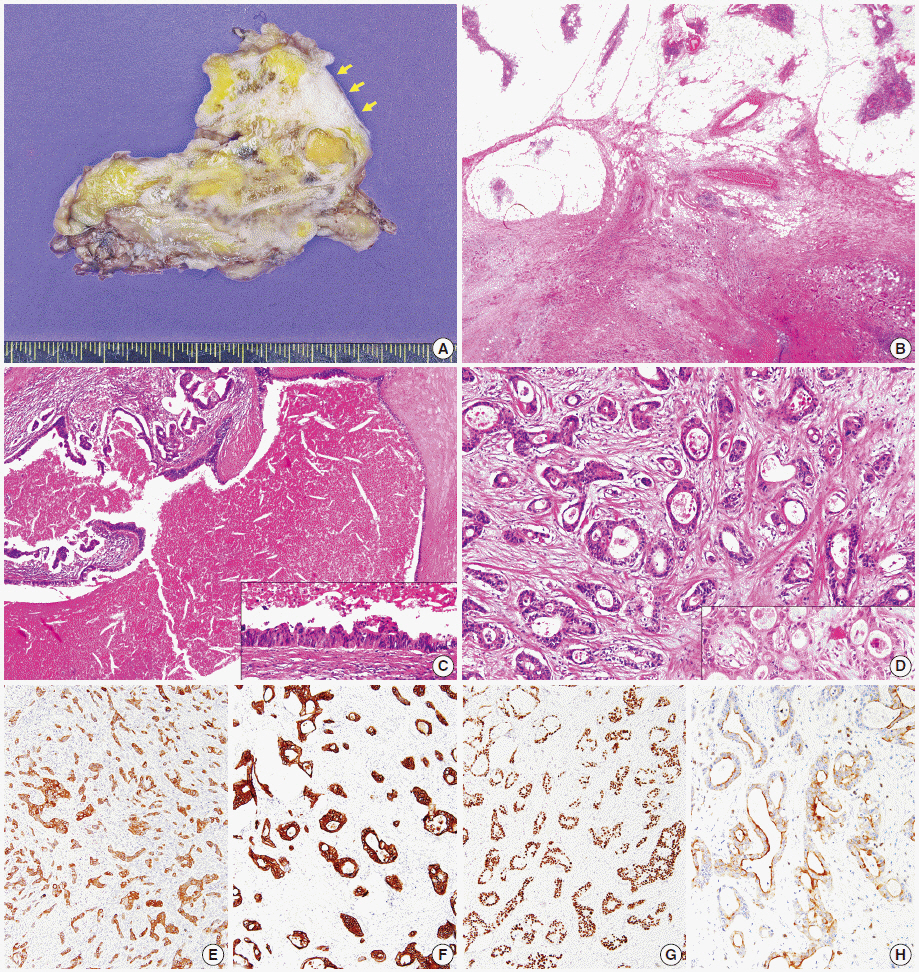
- Thymic carcinomas are uncommon malignant tumors, and thymic adenocarcinomas are extremely rare. Here, we describe a case of primary thymic adenocarcinoma in a 59-year-old woman. Histological examination of the tumor revealed tubular morphology with expression of cytokeratin 20 and caudal-type homeobox 2 according to immunohistochemistry, suggesting enteric features. Extensive clinical and radiological studies excluded the possibility of an extrathymic primary tumor. A review of the literature revealed only two global cases of primary tubular adenocarcinomas of the thymus with enteric immunophenotype.
MeSH Terms
Figure
Cited by 1 articles
-
Cytologic Characteristics of Thymic Adenocarcinoma with Enteric Differentiation: A Study of Four Fine-Needle Aspiration Specimens
Ah-Young Kwon, Joungho Han, Hae-yon Cho, Seokhwi Kim, Heejin Bang, Jiyeon Hyeon
J Pathol Transl Med. 2017;51(5):509-512. doi: 10.4132/jptm.2017.03.22.
Reference
-
1. Müller-Hermelink HK, Marx A, Kuo TT, Kurrer M, Chen G, Shimosato Y. Non-papillary adenocarcinomas. In : Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, editors. World Health Organization classification of tumours: pathology and genetics of tumours of the lung, pleura, thymus and heart. 3rd ed. Lyon: IARC Press;2004. p. 184.2. Moriyama S, Shimizu N, Kurita A, Teramoto S, Taguchi K. A case of adenocarcinoma of the thymus. Nihon Kyobu Geka Gakkai Zasshi. 1989; 37:717–22.3. Maghbool M, Ramzi M, Nagel I, et al. Primary adenocarcinoma of the thymus: an immunohistochemical and molecular study with review of the literature. BMC Clin Pathol. 2013; 13:17.
Article4. Matsuno Y, Morozumi N, Hirohashi S, Shimosato Y, Rosai J. Papillary carcinoma of the thymus: report of four cases of a new microscopic type of thymic carcinoma. Am J Surg Pathol. 1998; 22:873–80.5. Yoshino M, Hiroshima K, Motohashi S, et al. Papillary carcinoma of the thymus gland. Ann Thorac Surg. 2005; 80:741–2.
Article6. Furtado A, Nogueira R, Ferreira D, Tente D, Eisele R, Parente B. Papillary adenocarcinoma of the thymus: case report and review of the literature. Int J Surg Pathol. 2010; 18:530–3.
Article7. Choi WW, Lui YH, Lau WH, Crowley P, Khan A, Chan JK. Adenocarcinoma of the thymus: report of two cases, including a previously undescribed mucinous subtype. Am J Surg Pathol. 2003; 27:124–30.8. Ra SH, Fishbein MC, Baruch-Oren T, et al. Mucinous adenocarcinomas of the thymus: report of 2 cases and review of the literature. Am J Surg Pathol. 2007; 31:1330–6.9. Abdul-Ghafar J, Yong SJ, Kwon W, Park IH, Jung SH. Primary thymic mucinous adenocarcinoma: a case report. Korean J Pathol. 2012; 46:377–81.
Article10. Sawai T, Inoue Y, Doi S, et al. Tubular adenocarcinoma of the thymus: case report and review of the literature. Int J Surg Pathol. 2006; 14:243–6.
Article11. Misao T, Yamamoto Y, Nakano H, Toyooka S, Yamane M, Satoh K. Primary thymic adenocarcinoma with production of carbohydrate antigen 19-9 and carcinoembryonic antigen. Jpn J Thorac Cardiovasc Surg. 2004; 52:30–2.
Article12. Moser B, Schiefer AI, Janik S, et al. Adenocarcinoma of the thymus, enteric type: report of 2 cases, and proposal for a novel subtype of thymic carcinoma. Am J Surg Pathol. 2015; 39:541–8.13. Ishiwata T, Sekiya M, Suzuki T, Matsuoka T, Kumasaka T, Takahashi K. Thymic adenocarcinoma with sarcomatoid features characterized by intracaval tumor growth: report of a case. Surg Today. 2010; 40:1068–72.
Article14. Teramoto K, Kawaguchi Y, Hori T, et al. Thymic papillo-tubular adenocarcinoma containing a cyst: report of a case. Surg Today. 2012; 42:988–91.
Article15. Suster S, Rosai J. Thymic carcinoma: a clinicopathologic study of 60 cases. Cancer. 1991; 67:1025–32.
Article16. Wang S, Wang Z, Liu X, Wang D, Liu F. Prognostic factors of patients with thymic carcinoma after surgery: a retrospective analysis of 58 cases. World J Surg. 2014; 38:2032–8.
Article17. Filosso PL, Guerrera F, Rendina AE, et al. Outcome of surgically resected thymic carcinoma: a multicenter experience. Lung Cancer. 2014; 83:205–10.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytologic Characteristics of Thymic Adenocarcinoma with Enteric Differentiation: A Study of Four Fine-Needle Aspiration Specimens
- Mucinous Tubular and Spindle Cell Carcinoma of Kidney Occurring in a Patient with Pulmonary Adenocarcinoma
- A Case of Tubular Adenocarcinoma on Fistula of Duodenal Bulb
- A case of true thymic hyperplasia in the mediastinum with ectopic thymus in the neck
- A Case of Malignant Transformation of Gastric Tubular Adenoma Proven by 9-year Follow-Up