Immune Netw.
2011 Dec;11(6):364-367. 10.4110/in.2011.11.6.364.
Production of Prostaglandin E2 and I2 Is Coupled with Cyclooxygenase-2 in Human Follicular Dendritic Cells
- Affiliations
-
- 1Department of Microbiology and Immunology, School of Medicine, Kangwon National University, Chuncheon 200-701, Korea. jchoe@kangwon.ac.kr
- 2Department of Clinical Laboratory Science, Shinheung College, Uijeongbu 480-701, Korea.
- KMID: 2150721
- DOI: http://doi.org/10.4110/in.2011.11.6.364
Abstract
- BACKGROUND
Prostaglandins (PGs) play pathogenic and protective roles in inflammatory diseases. The novel concept of PGs as immune modulators is being documented by several investigators. By establishing an in vitro experimental model containing human follicular dendritic cell-like cells, HK cells, we reported that HK cells produce prostaglandin E2 (PGE2) and prostaglandin I2 (PGI2) and that these PGs regulate biological functions of T and B cells.
METHODS
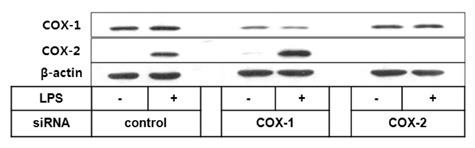
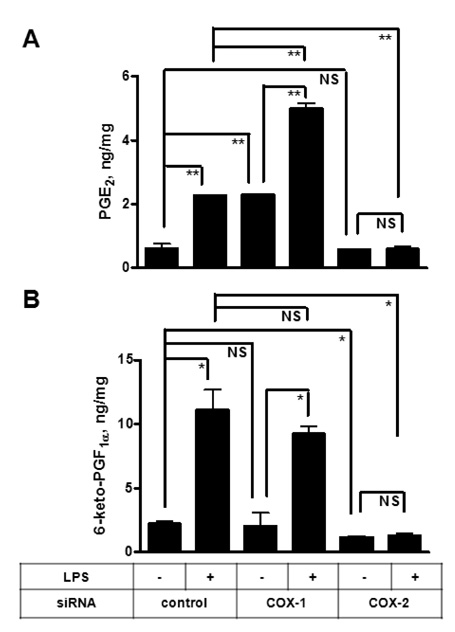
To investigate the respective contribution of cyclooxygenase-1 (COX-1) and COX-2 to PGE2 and PGI2 production in HK cells, we performed siRNA technology to knock down COX enzymes and examined the effect on PG production.
RESULTS
Both PGE2 and PGI2 productions were almost completely inhibited by the depletion of COX-2. In contrast, COX-1 knockdown did not significantly affect PG production induced by lipopolysaccharide (LPS).
CONCLUSION
The current results suggest that mPGES-1 and PGIS are coupled with COX-2 but not with COX-1 in human follicular dendritic cell (FDC) and may help understand the potential effects of selective COX inhibitors on the humoral immunity.
Keyword
MeSH Terms
-
Cyclooxygenase 1
Cyclooxygenase 2
Dendritic Cells, Follicular
Dinoprostone
Epoprostenol
Humans
Immunity, Humoral
Intramolecular Oxidoreductases
Models, Theoretical
Prostaglandins
Research Personnel
RNA, Small Interfering
Stromal Cells
Cyclooxygenase 1
Cyclooxygenase 2
Dinoprostone
Epoprostenol
Intramolecular Oxidoreductases
Prostaglandins
RNA, Small Interfering
Figure
Cited by 1 articles
-
IL-4 and HDAC Inhibitors Suppress Cyclooxygenase-2 Expression in Human Follicular Dendritic Cells
Whajung Cho, Seung Hee Hong, Jongseon Choe
Immune Netw. 2013;13(2):75-79. doi: 10.4110/in.2013.13.2.75.
Reference
-
1. Fosslien E. Cardiovascular complications of non-steroidal anti-inflammatory drugs. Ann Clin Lab Sci. 2005. 35:347–385.2. Ueno N, Takegoshi Y, Kamei D, Kudo I, Murakami M. Coupling between cyclooxygenases and terminal prostanoid synthases. Biochem Biophys Res Commun. 2005. 338:70–76.
Article3. Nakashima K, Ueno N, Kamei D, Tanioka T, Nakatani Y, Murakami M, Kudo I. Coupling between cyclooxygenases and prostaglandin F(2alpha) synthase. Detection of an inducible, glutathione-activated, membrane-bound prostaglandin F(2alpha)-synthetic activity. Biochim Biophys Acta. 2003. 1633:96–105.
Article4. Chandrasekharan S, Foley NA, Jania L, Clark P, Audoly LP, Koller BH. Coupling of COX-1 to mPGES1 for prostaglandin E2 biosynthesis in the murine mammary gland. J Lipid Res. 2005. 46:2636–2648.
Article5. Cheng Y, Wang M, Yu Y, Lawson J, Funk CD, Fitzgerald GA. Cyclooxygenases, microsomal prostaglandin E synthase-1, and cardiovascular function. J Clin Invest. 2006. 116:1391–1399.
Article6. Vane J, Corin RE. Prostacyclin: a vascular mediator. Eur J Vasc Endovasc Surg. 2003. 26:571–578.
Article7. Harizi H, Gualde N. The impact of eicosanoids on the crosstalk between innate and adaptive immunity: the key roles of dendritic cells. Tissue Antigens. 2005. 65:507–514.
Article8. Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002. 23:144–150.
Article9. Rocca B, FitzGerald GA. Cyclooxygenases and prostaglandins: shaping up the immune response. Int Immunopharmacol. 2002. 2:603–630.
Article10. Sreeramkumar V, Fresno M, Cuesta N. Prostaglandin E(2) and T cells: friends or foes? Immunol Cell Biol. 2001. [Epub ahead of print].11. Lee IY, Lee JH, Park WS, Nam EC, Shin YO, Choe JS. 3C8, a new monoclonal antibody directed against a follicular dendritic cell line, HK. Immune Netw. 2001. 1:26–31.
Article12. Lee IY, Ko EM, Kim SH, Jeoung DI, Choe J. Human follicular dendritic cells express prostacyclin synthase: a novel mechanism to control T cell numbers in the germinal center. J Immunol. 2005. 175:1658–1664.
Article13. Lee IY, Bae YD, Jeoung DI, Kang D, Park CH, Kim SH, Choe J. Prostacyclin production is not controlled by prostacyclin synthase but by cyclooxygenase-2 in a human follicular dendritic cell line, HK. Mol Immunol. 2007. 44:3168–3172.
Article14. Lee IY, Cho W, Kim J, Park CS, Choe J. Human follicular dendritic cells interact with T cells via expression and regulation of cyclooxygenases and prostaglandin E and I synthases. J Immunol. 2008. 180:1390–1397.
Article15. Cho W, Kim Y, Jeoung DI, Kim YM, Choe J. IL-4 and IL-13 suppress prostaglandins production in human follicular dendritic cells by repressing COX-2 and mPGES-1 expression through JAK1 and STAT6. Mol Immunol. 2011. 48:966–972.
Article16. Kim J, Park CS, Park CH, Jeoung DI, Kim YM, Choe J. Beraprost enhances the APC function of B cells by upregulating CD86 expression levels. J Immunol. 2011. 186:3866–3873.
Article17. Kim HS, Zhang X, Choi YS. Activation and proliferation of follicular dendritic cell-like cells by activated T lymphocytes. J Immunol. 1994. 153:2951–2961.18. Lee IY, Ha KS, Choe J. 3C8 antigen is a novel protein expressed by human follicular dendritic cells. Biochem Biophys Res Commun. 2003. 303:624–630.
Article19. Bolego C, Buccellati C, Prada A, Gaion RM, Folco G, Sala A. Critical role of COX-1 in prostacyclin production by human endothelial cells under modification of hydroperoxide tone. FASEB J. 2009. 23:605–612.
Article20. Spisni E, Bartolini G, Orlandi M, Belletti B, Santi S, Tomasi V. Prostacyclin (PGI2) synthase is a constitutively expressed enzyme in human endothelial cells. Exp Cell Res. 1995. 219:507–513.
Article21. Fosbol EL, Kober L, Torp-Pedersen C, Gislason GH. Cardiovascular safety of non-steroidal anti-inflammatory drugs among healthy individuals. Expert Opin Drug Saf. 2010. 9:893–903.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation between fertilization rate and human follicular fluid prostaglandin E2, prostaglandin F2a, prostaglandin E2: prostaglandin F2a ratio
- Prostaglandin E2 receptors as therapeutic targets in renal fibrosis
- IL-4 and HDAC Inhibitors Suppress Cyclooxygenase-2 Expression in Human Follicular Dendritic Cells
- Effect of high glucose on the prostaglandin E2 production in human gingival fibroblasts and periodontal ligament cells
- Expression of Cyclooxygenase - 2 in Intestinal Epithelial Cells in Response to Invasive Bacterial Infection and its Role of Epithelial Cell Apoptosis