Immune Netw.
2010 Apr;10(2):46-54. 10.4110/in.2010.10.2.46.
Roles of Host Nonhematopoietic Cells in Autoimmunity and Donor Cell Engraftment in Graft-versus-host Disease
- Affiliations
-
- 1Biomedical Research Center, Ulsan University Hospital, School of Medicine, Ulsan, Korea. bkwon@mail.ulsan.ac.kr
- 2School of Biological Sciences, University of Ulsan, Ulsan, Korea.
- 3Department of Pathology, Ulsan University Hospital, School of Medicine, University of Ulsan, Ulsan, Korea.
- 4Department of Surgery, Ulsan University Hospital, School of Medicine, University of Ulsan, Ulsan, Korea.
- KMID: 2150659
- DOI: http://doi.org/10.4110/in.2010.10.2.46
Abstract
-
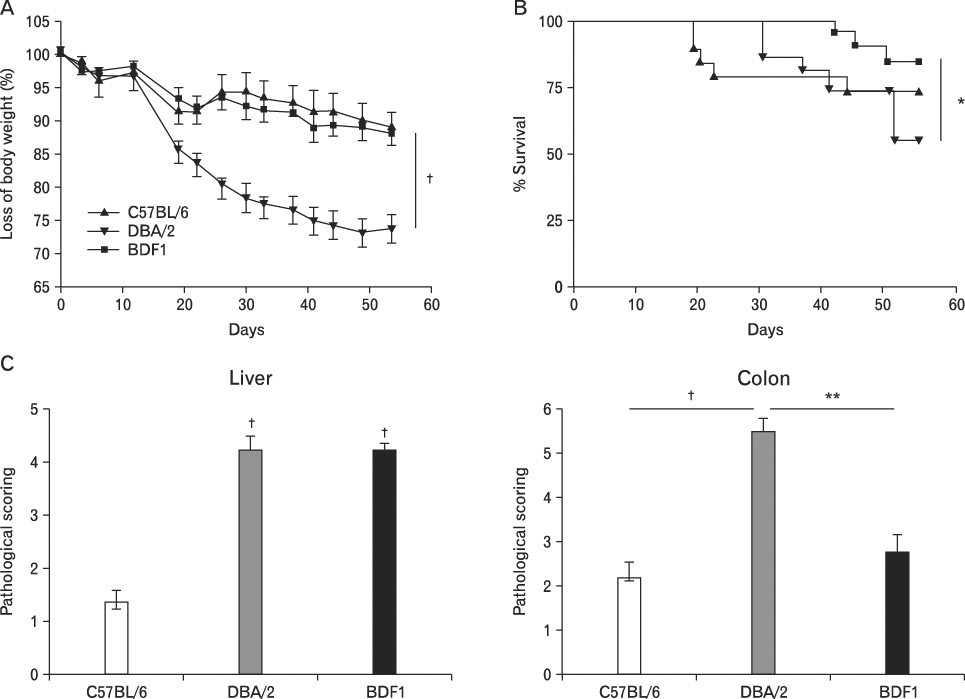
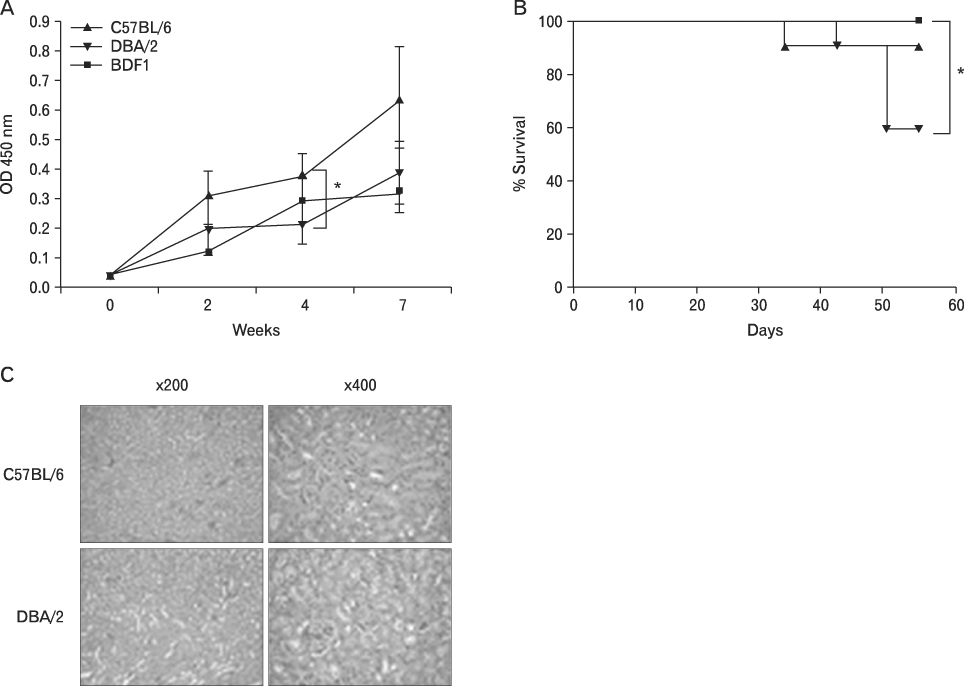
BACKGROUND: Graft-versus-host disease (GVHD) is initiated when alloreactive donor T cells are primed by host APCs to undergo clonal expansion and maturation. Since there is a controversy regarding the role of nonhematopoietic cells in GVHD, we wanted to investigate the influence of MHC disparity on nonhematopoietic cells on the pathogenesis of GVHD in the MHC-haplomismatched C57BL/6 (H-2(b)) or DBA/2 (H-2(d))-->unirradiated (C57BL/6xDBA/2) F(1)(BDF(1); H-2(b/d)) murine model of acute GVHD (aGVHD) or chronic GVHD (cGVHD).
METHODS
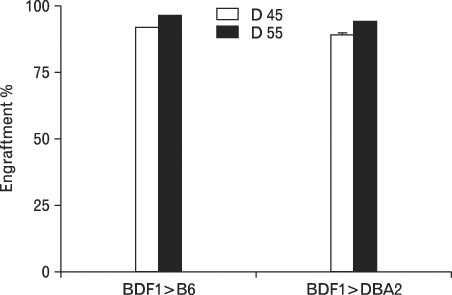
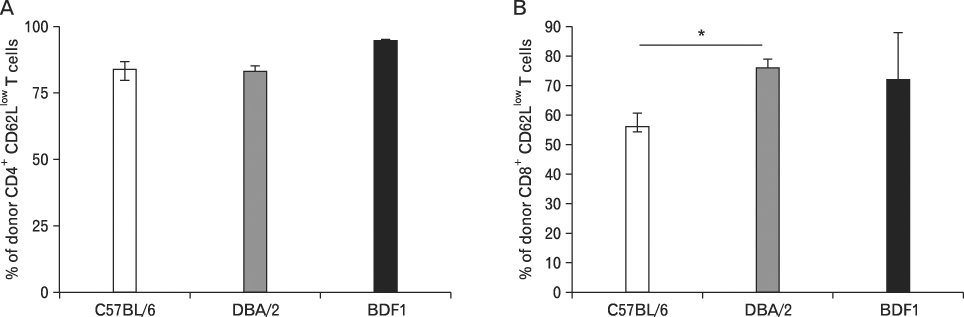
We generated (BDF(1)-->C57BL/6), (BDF(1)-->DBA/2), and (BDF(1)-->BDF(1)) chimeras and examined GVHD-related parameters and donor cell engraftment in those chimeras.
RESULTS
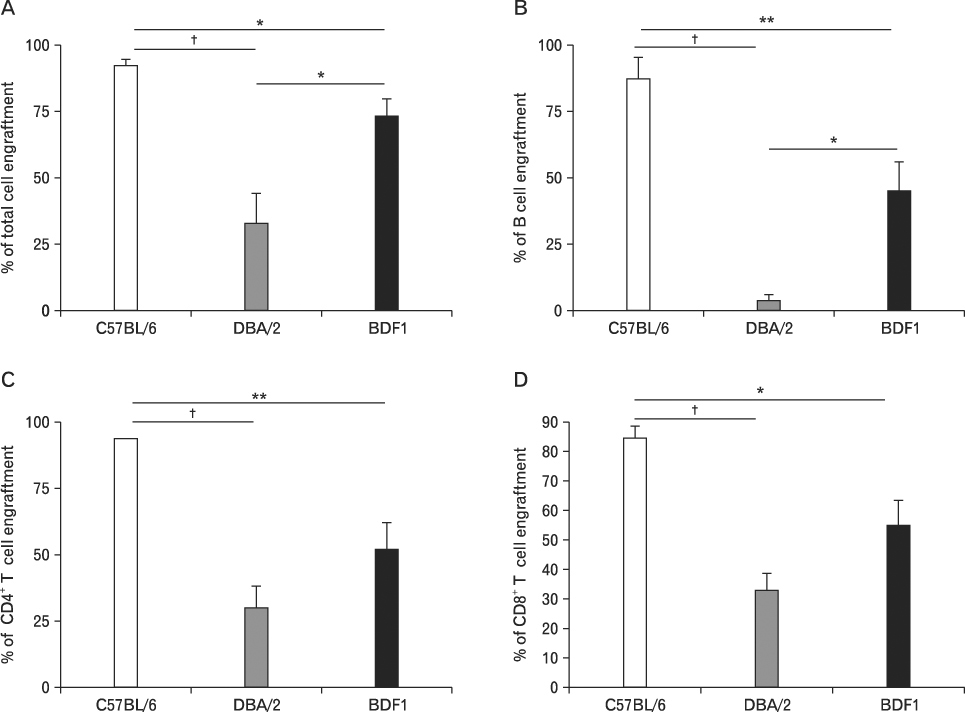
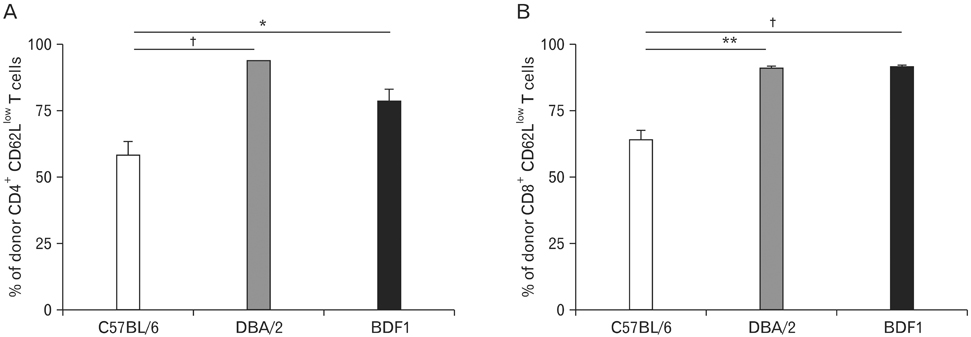
Using this experimental system, we found that 1) severe aGVHD across MHC Ag barrier depends on the expression of nonhematopoietically rather than hematopoietically derived alloAgs for maximal GVHD manifestations; 2) host APCs were sufficient to break B cell tolerance to self molecules in cGVHD, whereas host APCs were insufficient to induce autoimmunity in aGVHD; 3) donor cell engraftment was greatly enhanced in the host with MHC-matched nonhematopoietic cells.
CONCLUSION
Taken together, our results provide an insight into how MHC disparity on GVHD target organs contribute to the pathogenesis of GVHD.
Figure
Reference
-
1. Murphy WJ. Revisiting graft-versus-host disease models of autoimmunity: new insights in immune regulatory processes. J Clin Invest. 2000. 106:745–747.
Article2. Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007. 25:139–170.
Article3. Via CS, Sharrow SO, Shearer GM. Role of cytotoxic T lymphocytes in the prevention of lupus-like disease disease occurring in a murine model of graft-vs-host disease. J Immunol. 1987. 139:1840–1849.4. Rus V, Svetic A, Nguyen P, Gause WC, Via CS. Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft-versus-host disease. J Immunol. 1995. 155:2396–2406.5. Kim J, Choi WS, Kang H, Kim HJ, Suh J-H, Sakguchi S, Kwon B. Conversion of alloantigen-specific CD8+ T cell anergy to CD8+ T cell priming through in vivo ligation of glucocorticoid-induced TNF receptor. J Immunol. 2006. 176:5223–5231.
Article6. Kim J, Park K, Kim HJ, Kim J, Kim H-A, Jung D, Kim HJ, Choi H-J, Choi S-K, Suh K, Cho HR, Kwon B. Breaking of CD8+ T cell tolerance through in vivo ligation of CD40 results in inhibition of chronic graft-versus-host disease and complete donor cell engraftment. J Immunol. 2008. 181:7380–7389.
Article7. Via CS, Finkelman FD. Critical role of interleukin-2 in the development of acute graftversus-host disease. Int Immunol. 1993. 5:565–572.
Article8. Shustov A, Luzina I, Nguyen P, Papadimitriou JP, Handwerger B, Elkon KB, Via CS. Role of perforin in controlling B-cell hyperactivity and humoral autoimmunity. J Clin Invest. 2000. 106:R39–R47.
Article9. Via CS, Shustov A, Rus V, Lang T, Nguyen P, Finkelman FD. In vivo neutralization of TNF-α promotes humoral autoimmunity by preventing the induction of CTL. J Immunol. 2001. 167:6821–6826.
Article10. Aosai F, Ohlen C, Ljunggren HG, Höqlund P, Franksson L, Ploegh H, Towsend A, Kärre K, Stauss HJ. Different types of allospecific CTL clones identified by their ability to recognize peptide loading-defective target cells. Eur J Immunol. 1991. 21:2767–2774.
Article11. Man S, Saler RD, Engelhard VH. Role of endogenous peptide in human alloreactive cytotoxic T cell responses. Int Immunol. 1992. 4:367–375.
Article12. Crumpacker DB, Alexander J, Cresswell P, Engelhard VH. Role of endogenous peptides in murine allogeneic cytotoxic T cell responses assessed using transfectants of the antigen-processing mutant 174xCEM.T2. J Immunol. 1992. 148:3004–3011.13. Teshima T, Ordemann R, Reddy P, Gagins S, Liu C, Cooke KR, Ferrara JL. Acute graft-vs-host disease does not required alloantigen expression on host epithelium. Nat Med. 2002. 8:575–581.
Article14. Kim J, Choi WS, La S, Suh J-H, Kim B-S, Cho HR, Kwon BS, Kwon B. Stimulation with 4-1BB inhibits chronic graft-versus-host disease by inducing activation-induced cell death of donor CD4+ T cells. Blood. 2005. 105:2206–2213.
Article15. Kim J, Choi WS, Kim HJ, Kwon B. Prevention of chronic graft-versus-host disease by stimulation with glucocorticoid-induced TNF receptor. Exp Mol Med. 2006. 38:94–99.
Article16. Kim J, Kim HJ, Choi WS, Cho HR, Kwon B. Maintenance of CD8+ T-cell anergy by CD4+CD25+ regulatory T cells. Exp Mol Med. 2006. 38:494–501.
Article17. Cooke KR, Krenger W, Martin TR, Kobzik L, Brewer J, Simmons R, Crawford JM, van den Brink MR, Ferra JL. Host reactive donor T cells are associated with lung injury after experimental allogeneic bone marrow transplantation. Blood. 1998. 92:2571–2580.
Article18. Hill GR, Crawford JM, Cooke KR, Brinson YS, Pal L, Ferrara JL. Total body irradiation effects on acute graft versus host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997. 90:3204–3213.
Article19. Korngold R, Sprent J. Features of T cells causing H-2-restricted lethal graft-vs-host disease across minor histocompatibility barriers. J Exp Med. 1982. 155:872–883.
Article20. Jones SC, Murphy GF, Friedman TM, Korngold R. Importance of donor histocompatibility antigen expression by nonhematopoietic tissues in a CD4+ T cell mediated graft-versus-host disease. J Clin Invest. 2003. 112:1880–1886.
Article21. Chu Y-W, Gress RE. Murine models of chronic graft-versus-host disease: insights and unresolved issues. Biol Blood Marrow Transplant. 2008. 14:365–378.
Article22. Horowitz JB, Kaye J, Katz ME, Janeway CA Jr. Ability of fixed B-lymphoma cells to present foreign protein antigen graftments and allogeneic MHC molecules to a cloned helper-T cell line. Cell Immunol. 1987. 109:429–436.
Article23. Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, Shlomchik MJ, Emerson SG. Prevention of graft versus host disease by inactivation of host antigen presenting cells. Science. 1999. 285:412–415.
Article24. Zhang Y, Hexner E, Frank D, Emerson SG. CD4+ T cells de novo from donor hemopoietic stem cells mediate the evolution from acute to chronic graft-versus-host disease. J Immunol. 2007. 179:3305–3314.
Article25. Benichou G, Takizawa PA, Olson CA, McMillian M, Secarz EE. Donor major histocompatibility complex (MHC) peptides are presented by recipient MHC molecules during graft rejection. J Exp Med. 1992. 175:305–308.
Article26. Liu Z, Braunstein NS, Suciu-Foca N. T cell recognition of allopeptides in context of syngeneic MHC. J Immunol. 1992. 148:35–40.
Article27. Matte CC, Liu J, Anderson BE, Athanasiadis I, Jain D, McNiff J, Shlomchik WD. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004. 10:987–992.
Article28. Song HK, Noirchashm H, Lieu YK, Rostani S, Greeley SA, Barker CF, Naji A. Cutting edge: alloimmune responses against major and minor histocompatibility antigens: distinct division kinetics and requirement for CD28 costimulation. J Immunol. 1999. 162:2467–2471.29. Cooke KR, Hill GR, Crawford JM, Bungard D, Brinson YS, Delmonte J Jr, Ferrara JL. Tumor necrosis factor-alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest. 1998. 102:1882–1891.
Article30. Krijianouski OI, Hill GR, Cooke KR, Teshima T, Crawford JM, Brinson YS, Ferrara JL. Keratinocyte growth factor separates graft-versus-leukemia effects from graft-versushost disease. Blood. 1999. 94:825–831.
Article31. Reddy P, Teshima T, Kukuruga M, Ordemann R, Liu C, Lowler K, Ferrara JL. Interleukin-18 regulates acute graft-versus-host disease by enhancing Fas-mediated donor T cell apoptosis. J Exp Med. 2001. 194:1433–1440.
Article32. Brown GR, Lee E, Thiele DL. TNF-TNFR2 interactions are critical for the development of intestinal graft-versus-host disease in MHC class II disparate (C57BL/6J→C57BL/6J×bm12)F1 mice. J Immunol. 2002. 168:3065–3071.
Article33. Gonzalez M, Quezada SA, Blazar BR, Panoskaltsis-Mortari A, Rudensky AY, Noelle RJ. The balance between donor T cell anergy and suppression versus lethal graft-versus-host disease is determined by host conditioning. J Immunol. 2002. 169:5581–5589.
Article34. Kim J, Kim HJ, Park K, Kim J, Choi HJ, Yagita H, Nam SK, Cho HR, Kwon B. Costimulatory molecule-targeted immunotherapy of chronic graft-versus-host disease. Blood. 2007. 110:776–782.
Article35. Kim W, Kim J, Jung D, Kim H, Choi HJ, Cho HR, Kwon B. Induction of lethal graft-versus-host disease by anti-CD137 monoclonal antibody in mice prone to chronic GVHD. Biol Blood Marrow Transplant. 2009. 15:306–314.
Article36. Rouquette-Gally AM, Boyeldieu D, Gluckman E, Abuaf N, Combrisson A. Autoimmunity in 28 patients after allogeneic bone marrow transplantation: comparison with Sjogren syndrome and scleroderma. Br J Haematol. 1987. 66:45–47.
Article37. Rouquette-Gally AM, Boyeldieu D, Prost AC, Gluckman E. Autoimmunity after allogeneic bone marrow transplantation. A study of 53 long-term-surviving patients. Transplantation. 1988. 46:238–240.
Article38. Sherer Y, Shoenfeld Y. Autoimmune disease and autoimmunity post-bone marrow transplantation. Bone Marrow Transplant. 1998. 22:873–881.
Article39. Tivol E, Komorowski R, Drobyski WR. Emergent autoimmuity in graft-versus-host disease. Blood. 2005. 105:4885–4891.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute Cutaneous Graft-Versus-Host Reaction
- Intervention with costimulatory pathways as a therapeutic approach for graft-versus-host disease
- CTLA-4-Tg/CD-28-KO Mice Exhibit Reduced T Cell Proliferation in vivo Compared to CD-28-KO Mice in a Graft-versus-host Disease Model
- A Histopathological Comparative Study between Erythema Multiforme and Acute Cutaneous Graft - versus - Host Reactions
- Graft-versus-Leukemia Effect of Nonmyeloablative Stem Cell Transplantation