Allergy Asthma Immunol Res.
2015 Sep;7(5):421-430. 10.4168/aair.2015.7.5.421.
Chronic Rhinosinusitis and the Coagulation System
- Affiliations
-
- 1Division of Allergy and Immunology, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA. rpschleimer@northwestern.edu
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 3Division of Rheumatology, Department of Medicine, Kyung Hee University School of Medicine, Seoul, Korea.
- 4Division of Otorhinolaryngology Head and Neck Surgery, Department of Sensory and Locomotor Medicine, University of Fukui, Fukui, Japan.
- KMID: 2147950
- DOI: http://doi.org/10.4168/aair.2015.7.5.421
Abstract
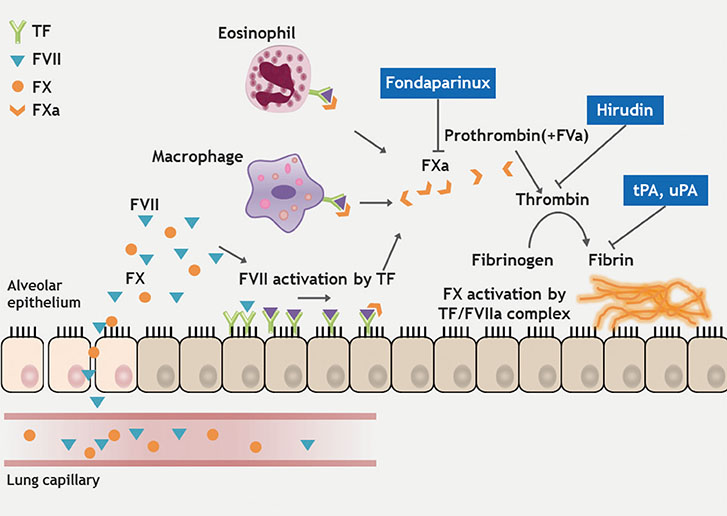
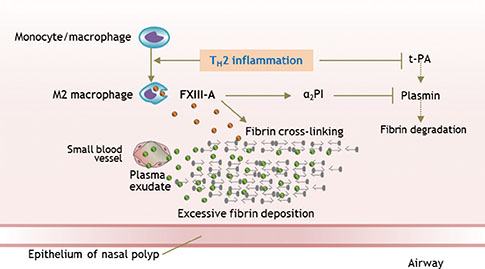
- Chronic rhinosinusitis (CRS) is one of the most common chronic diseases in adults and severely affects quality of life in patients. Although various etiologic and pathogenic mechanisms of CRS have been proposed, the causes of CRS remain uncertain. Abnormalities in the coagulation cascade may play an etiologic role in many diseases, such as asthma and other inflammatory conditions. While studies on the relationship between asthma and dysregulated coagulation have been reported, the role of the coagulation system in the pathogenesis of CRS has only been considered following recent reports. Excessive fibrin deposition is seen in nasal polyp (NP) tissue from patients with chronic rhinosinusitis with nasal polyp (CRSwNP) and is associated with activation of thrombin, reduction of tissue plasminogen activator (t-PA) and upregulation of coagulation factor XIII-A (FXIII-A), all events that can contribute to fibrin deposition and crosslinking. These findings were reproduced in a murine model of NP that was recently established. Elucidation of the mechanisms of fibrin deposition may enhance our understanding of tissue remodeling in the pathophysiology of NP and provide new targets for the treatment of CRSwNP.
Keyword
MeSH Terms
Figure
Reference
-
1. Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. Otolaryngol Head Neck Surg. 2004; 131:S1–S62.2. Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol. 2008; 22:549–559.3. Schleimer RP, Kato A, Peters A, Conley D, Kim J, Liu MC, et al. Epithelium, inflammation, and immunity in the upper airways of humans: studies in chronic rhinosinusitis. Proc Am Thorac Soc. 2009; 6:288–294.4. Van Bruaene N, Derycke L, Perez-Novo CA, Gevaert P, Holtappels G, De Ruyck N, et al. TGF-beta signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol. 2009; 124:253–259. 259.e1–259.e2.5. Bachert C, Gevaert P, van Cauwenberge P. Staphylococcus aureus superantigens and airway disease. Curr Allergy Asthma Rep. 2002; 2:252–258.6. Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006; 61:1280–1289.7. Gabazza EC, Osamu T, Yamakami T, Ibata H, Sato T, Sato Y, et al. Correlation between clotting and collagen metabolism markers in rheumatoid arthritis. Thromb Haemost. 1994; 71:199–202.8. Neale TJ, Tipping PG, Carson SD, Holdsworth SR. Participation of cell-mediated immunity in deposition of fibrin in glomerulonephritis. Lancet. 1988; 2:421–424.9. Hudson M, Hutton RA, Wakefield AJ, Sawyerr AM, Pounder RE. Evidence for activation of coagulation in Crohn's disease. Blood Coagul Fibrinolysis. 1992; 3:773–778.10. Szaba FM, Smiley ST. Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood. 2002; 99:1053–1059.11. de Boer JD, Majoor CJ, van't Veer C, Bel EH, van der Poll T. Asthma and coagulation. Blood. 2012; 119:3236–3244.12. Lambrecht BN, Hammad H. Asthma and coagulation. N Engl J Med. 2013; 369:1964–1966.13. Millien VO, Lu W, Shaw J, Yuan X, Mak G, Roberts L, et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science. 2013; 341:792–796.14. Ciprandi G, Caimmi D, Miraglia Del, La Rosa M, Salpietro C, Marseglia GL. Recent developments in united airways disease. Allergy Asthma Immunol Res. 2012; 4:171–177.15. Lee SY, Yoon SH, Song WJ, Lee SH, Kang HR, Kim SS, et al. Influence of chronic sinusitis and nasal polyp on the lower airway of subjects without lower airway diseases. Allergy Asthma Immunol Res. 2014; 6:310–315.16. Shimizu S, Gabazza EC, Ogawa T, Tojima I, Hoshi E, Kouzaki H, et al. Role of thrombin in chronic rhinosinusitis-associated tissue remodeling. Am J Rhinol Allergy. 2011; 25:7–11.17. Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, et al. Excessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expression. Am J Respir Crit Care Med. 2013; 187:49–57.18. Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, et al. Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2013; 132:584–592.e4.19. Nesheim M. Thrombin and fibrinolysis. Chest. 2003; 124:33S–39S.20. Schenone M, Furie BC, Furie B. The blood coagulation cascade. Curr Opin Hematol. 2004; 11:272–277.21. Schuliga M, Westall G, Xia Y, Stewart AG. The plasminogen activation system: new targets in lung inflammation and remodeling. Curr Opin Pharmacol. 2013; 13:386–393.22. Levi M, Schultz MJ, Rijneveld AW, van der Poll T. Bronchoalveolar coagulation and fibrinolysis in endotoxemia and pneumonia. Crit Care Med. 2003; 31:S238–S242.23. Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010; 38:S26–S34.24. Wygrecka M, Jablonska E, Guenther A, Preissner KT, Markart P. Current view on alveolar coagulation and fibrinolysis in acute inflammatory and chronic interstitial lung diseases. Thromb Haemost. 2008; 99:494–501.25. Kornerup KN, Page CP. The role of platelets in the pathophysiology of asthma. Platelets. 2007; 18:319–328.26. Yamamoto H, Nagata M, Tabe K, Kimura I, Kiuchi H, Sakamoto Y, et al. The evidence of platelet activation in bronchial asthma. J Allergy Clin Immunol. 1993; 91:79–87.27. Kowal K, Pampuch A, Kowal-Bielecka O, DuBuske LM, Bodzenta-Łukaszyk A. Platelet activation in allergic asthma patients during allergen challenge with Dermatophagoides pteronyssinus. Clin Exp Allergy. 2006; 36:426–432.28. Gresele P, Dottorini M, Selli ML, Iannacci L, Canino S, Todisco T, et al. Altered platelet function associated with the bronchial hyperresponsiveness accompanying nocturnal asthma. J Allergy Clin Immunol. 1993; 91:894–902.29. Knauer KA, Lichtenstein LM, Adkinson NF Jr, Fish JE. Platelet activation during antigen-induced airway reactions in asthmatic subjects. N Engl J Med. 1981; 304:1404–1407.30. Perrio MJ, Ewen D, Trevethick MA, Salmon GP, Shute JK. Fibrin formation by wounded bronchial epithelial cell layers in vitro is essential for normal epithelial repair and independent of plasma proteins. Clin Exp Allergy. 2007; 37:1688–1700.31. Wagers SS, Norton RJ, Rinaldi LM, Bates JH, Sobel BE, Irvin CG. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J Clin Invest. 2004; 114:104–111.32. Gabazza EC, Taguchi O, Tamaki S, Takeya H, Kobayashi H, Yasui H, et al. Thrombin in the airways of asthmatic patients. Lung. 1999; 177:253–262.33. Kanazawa H, Yoshikawa T. Up-regulation of thrombin activity induced by vascular endothelial growth factor in asthmatic airways. Chest. 2007; 132:1169–1174.34. Terada M, Kelly EA, Jarjour NN. Increased thrombin activity after allergen challenge: a potential link to airway remodeling? Am J Respir Crit Care Med. 2004; 169:373–377.35. Schouten M, van de Pol M, Levi M, van der Poll T, van der Zee J. Early activation of coagulation after allergen challenge in patients with allergic asthma. J Thromb Haemost. 2009; 7:1592–1594.36. Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004; 114:997–1008.37. Shimizu S, Shimizu T, Morser J, Kobayashi T, Yamaguchi A, Qin L, et al. Role of the coagulation system in allergic inflammation in the upper airways. Clin Immunol. 2008; 129:365–371.38. Shimizu S, Gabazza EC, Hayashi T, Ido M, Adachi Y, Suzuki K. Thrombin stimulates the expression of PDGF in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2000; 279:L503–L510.39. Hauck RW, Schulz C, Schömig A, Hoffman RK, Panettieri RA Jr. α-Thrombin stimulates contraction of human bronchial rings by activation of protease-activated receptors. Am J Physiol. 1999; 277:L22–L29.40. Epstein MM. Do mouse models of allergic asthma mimic clinical disease? Int Arch Allergy Immunol. 2004; 133:84–100.41. Hogan KA, Weiler H, Lord ST. Mouse models in coagulation. Thromb Haemost. 2002; 87:563–574.42. Shinagawa K, Ploplis VA, Castellino FJ. A severe deficiency of coagulation factor VIIa results in attenuation of the asthmatic response in mice. Am J Physiol Lung Cell Mol Physiol. 2009; 296:L763–L770.43. Shinagawa K, Martin JA, Ploplis VA, Castellino FJ. Coagulation factor Xa modulates airway remodeling in a murine model of asthma. Am J Respir Crit Care Med. 2007; 175:136–143.44. Oh CK, Ariue B, Alban RF, Shaw B, Cho SH. PAI-1 promotes extracellular matrix deposition in the airways of a murine asthma model. Biochem Biophys Res Commun. 2002; 294:1155–1160.45. Cho SH, Hall IP, Wheatley A, Dewar J, Abraha D, Del Mundo J, et al. Possible role of the 4G/5G polymorphism of the plasminogen activator inhibitor 1 gene in the development of asthma. J Allergy Clin Immunol. 2001; 108:212–214.46. Cho S, Kang J, Lyttle C, Harris K, Daley B, Grammer L, et al. Association of elevated plasminogen activator inhibitor 1 levels with diminished lung function in patients with asthma. Ann Allergy Asthma Immunol. 2011; 106:371–377.47. Xiao W, Hsu YP, Ishizaka A, Kirikae T, Moss RB. Sputum cathelicidin, urokinase plasminogen activation system components, and cytokines discriminate cystic fibrosis, COPD, and asthma inflammation. Chest. 2005; 128:2316–2326.48. Miyamoto S, Hattori N, Senoo T, Onari Y, Iwamoto H, Kanehara M, et al. Intra-airway administration of small interfering RNA targeting plasminogen activator inhibitor-1 attenuates allergic asthma in mice. Am J Physiol Lung Cell Mol Physiol. 2011; 301:L908–L916.49. Lee SH, Eren M, Vaughan DE, Schleimer RP, Cho SH. A plasminogen activator inhibitor-1 inhibitor reduces airway remodeling in a murine model of chronic asthma. Am J Respir Cell Mol Biol. 2012; 46:842–846.50. Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med. 2003; 31:S213–S220.51. Del Rosso M, Fibbi G, Pucci M, Margheri F, Serrati S. The plasminogen activation system in inflammation. Front Biosci. 2008; 13:4667–4686.52. Pawliczak R, Lewandowska-Polak A, Kowalski ML. Pathogenesis of nasal polyps: an update. Curr Allergy Asthma Rep. 2005; 5:463–471.53. Seshadri S, Lin DC, Rosati M, Carter RG, Norton JE, Suh L, et al. Reduced expression of antimicrobial PLUNC proteins in nasal polyp tissues of patients with chronic rhinosinusitis. Allergy. 2012; 67:920–928.54. Bagoly Z, Katona E, Muszbek L. Factor XIII and inflammatory cells. Thromb Res. 2012; 129:Suppl 2. S77–S81.55. Derrick EK, Barker JN, Khan A, Price ML, Macdonald DM. The tissue distribution of factor XIIIa positive cells. Histopathology. 1993; 22:157–162.56. Katona E E, Ajzner E, Tóth K, Kárpáti L, Muszbek L. Enzyme-linked immunosorbent assay for the determination of blood coagulation factor XIII A-subunit in plasma and in cell lysates. J Immunol Methods. 2001; 258:127–135.57. Malara A, Gruppi C, Rebuzzini P, Visai L, Perotti C, Moratti R, et al. Megakaryocyte-matrix interaction within bone marrow: new roles for fibronectin and factor XIII-A. Blood. 2011; 117:2476–2483.58. Adány R, Bárdos H. Factor XIII subunit A as an intracellular transglutaminase. Cell Mol Life Sci. 2003; 60:1049–1060.59. Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É. Factor XIII: a coagulation factor with multiple plasmatic and cellular functions. Physiol Rev. 2011; 91:931–972.60. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008; 8:958–969.61. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010; 32:593–604.62. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011; 11:723–737.63. Fairweather D, Cihakova D. Alternatively activated macrophages in infection and autoimmunity. J Autoimmun. 2009; 33:222–230.64. Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008; 13:453–461.65. Töröcsik D, Szeles L, Paragh G Jr, Rakosy Z, Bardos H, Nagy L, et al. Factor XIII-A is involved in the regulation of gene expression in alternatively activated human macrophages. Thromb Haemost. 2010; 104:709–717.66. Kim DW, Khalmuratova R, Hur DG, Jeon SY, Kim SW, Shin HW, et al. Staphylococcus aureus enterotoxin B contributes to induction of nasal polypoid lesions in an allergic rhinosinusitis murine model. Am J Rhinol Allergy. 2011; 25:e255–e261.67. Gabazza EC, Taguchi O, Kamada H, Hayashi T, Adachi Y, Suzuki K. Progress in the understanding of protease-activated receptors. Int J Hematol. 2004; 79:117–122.68. Ramachandran R, Hollenberg MD. Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol. 2008; 153:Suppl 1. S263–S282.69. Mercer PF, Johns RH, Scotton CJ, Krupiczojc MA, Königshoff M, Howell DC, et al. Pulmonary epithelium is a prominent source of proteinase-activated receptor-1-inducible CCL2 in pulmonary fibrosis. Am J Respir Crit Care Med. 2009; 179:414–425.70. Hayashi S, Takeuchi K, Suzuki S, Tsunoda T, Tanaka C, Majima Y. Effect of thrombin on permeability of human epithelial cell monolayers. Pharmacology. 2006; 76:46–52.71. Feistritzer C, Mosheimer BA, Kaneider NC, Riewald M, Patsch JR, Wiedermann CJ. Thrombin affects eosinophil migration via protease-activated receptor-1. Int Arch Allergy Immunol. 2004; 135:12–16.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medical treatment according to phenotypes of chronic rhinosinusitis
- The Clinical Significance of Lund-Mackay CT Staging System in Assessing the Severity of Chronic Rhinosinusitis
- Clinical Characteristics and Treatment of Fungal Rhinosinusitis
- The Role of Allergy in the Severity of Chronic Rhinosinusitis
- Mechanisms of Glucocorticoid Action in Chronic Rhinosinusitis