J Korean Surg Soc.
2010 Oct;79(4):294-299. 10.4174/jkss.2010.79.4.294.
Effect of Nanoparticle with VEGF in Mouse Ischemic Hindlimb Model
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. jwhamd@snu.ac.kr
- KMID: 2145032
- DOI: http://doi.org/10.4174/jkss.2010.79.4.294
Abstract
- PURPOSE
Vascular endothelial growth factor (VEGF) is one of the factors regulating angiogenesis. For angiogenesis, the local concentration of VEGF has to be maintained. Because of its short half-life, VEGF has been conjugated with nanoparticles. Some nanoparticles, such as poly (lactic-co-glycolic acid (PLGA)) or polyethylenimine (PEI) are commonly used in this field, but have weak points such as faster release than expected and cell toxicity. We investigated the effect of core/shell nanoparticles including lecithin lipid cores in the ischemic hindlimb model.
METHODS
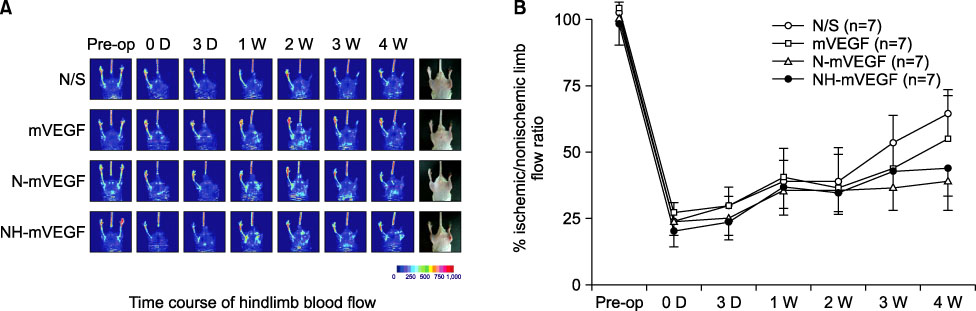
Mice were anesthetized and a region of the common femoral artery and vein was ligated and excised. Hindlimb ischemic mice (n=28) were divided randomly into four groups: Control group (normal saline, n=7), mouse VEGF group (mVEGF, n=7), nanoparticle including mVEGF group (N-mVEGF, n=7), and nanoparticle/hydrogel mouse VEGF group (NH-mVEGF, n=7). The drug was injected postoperatively into the thigh muscle of the ischemic limb. Perfusion, capillary number and H&E stain were assessed 28 d after treatment.
RESULTS
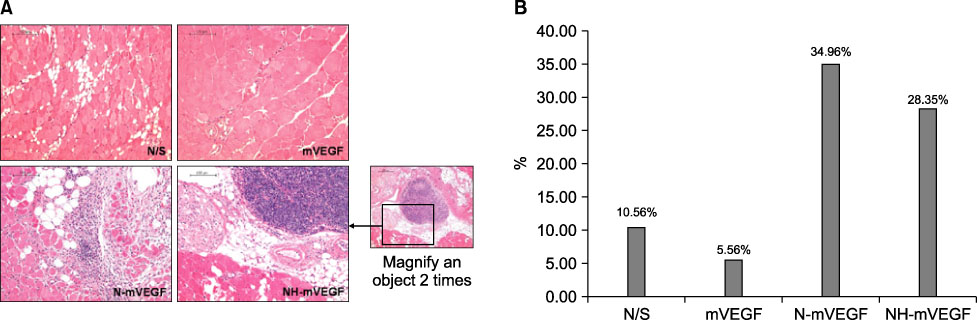
The capillary number increased in N-mVEGF and mVEGF group (P=0.026). Improvements of ischemic limb perfusion were inferior in N-mVEGF, NH-mVEGF groups (P=0.006) compared to other groups. Mice received N-mVEGF, NH-mVEGF treatment showed significant inflammation in the H&E staining.
CONCLUSION
Sustained VEGF delivery via core/shell nanoparticle with lecithin core did not show improved perfusion rate despite an increase in capillary number. Furthermore, vacuolization and induction of inflammation requiring a different composition of nanoparticle should be tested.
MeSH Terms
Figure
Reference
-
1. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006. 113:e463–e654.2. Hall H. Modified fibrin hydrogel matrices: both, 3D-scaffolds and local and controlled release systems to stimulate angiogenesis. Curr Pharm Des. 2007. 13:3597–3607.3. Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999. 5:1359–1364.4. Folkman J. Diagnostic and therapeutic applications of angiogenesis research. C R Acad Sci III. 1993. 316:909–918.5. Ware JA, Simons M. Angiogenesis in ischemic heart disease. Nat Med. 1997. 3:158–164.6. Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003. 2:863–871.7. Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. The VIVA trial: vascular endothelial growth factor in Ischemia for vascular angiogenesis. Circulation. 2003. 107:1359–1365.8. Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001. 42:2408–2413.9. Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003. 23:117–145.10. Yang R, Ogasawara AK, Zioncheck TF, Ren Z, He GW, DeGuzman GG, et al. Exaggerated hypotensive effect of vascular endothelial growth factor in spontaneously hypertensive rats. Hypertension. 2002. 39:815–820.11. Oh KS, Han SK, Lee HS, Koo HM, Kim RS, Lee KE, et al. Core/Shell nanoparticles with lecithin lipid cores for protein delivery. Biomacromolecules. 2006. 7:2362–2367.12. Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000. 21:2521–2527.13. Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc Pathol. 2003. 12:295–310.14. Suri SS, Fenniri H, Singh B. Nanotechnology-based drug delivery systems. J Occup Med Toxicol. 2007. 2:16.15. Abdallah B, Hassan A, Benoist C, Goula D, Behr JP, Demeneix BA. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum Gene Ther. 1996. 7:1947–1954.16. Godbey WT, Wu KK, Mikos AG. Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J Biomed Mater Res. 1999. 45:268–275.17. Benns JM, Maheshwari A, Furgeson DY, Mahato RI, Kim SW. Folate-PEG-folate-graft-polyethylenimine-based gene delivery. J Drug Target. 2001. 9:123–139.18. Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 2002. 16:1217–1226.19. Stern M, Ulrich K, Geddes DM, Alton EW. Poly (D, L-lactide-co-glycolide)/DNA microspheres to facilitate prolonged transgene expression in airway epithelium in vitro, ex vivo and in vivo. Gene Ther. 2003. 10:1282–1288.20. Jeon O, Kang SW, Lim HW, Chung JH, Kim BS. Long-term and zero-order release of basic fibroblast growth factor from heparin-conjugated poly(L-lactide-co-glycolide) nanospheres and fibrin gel. Biomaterials. 2006. 27:1598–1607.21. Dai C, Wang B, Zhao H. Microencapsulation peptide and protein drugs delivery system. Colloids Surf B Biointerfaces. 2005. 41:117–120.22. Sinha VR, Trehan A. Biodegradable microspheres for protein delivery. J Control Release. 2003. 90:261–280.23. Robertson D, Hellweg T, Tiersch B, Koetz J. Polymer-induced structural changes in lecithin/sodium dodecyl sulfate-based multilamellar vesicles. J Colloid Interface Sci. 2004. 270:187–194.24. Yang Z, von Ballmoos MW, Diehm N, Baumgartner I, Kalka C, Di Santo S. Call for a reference model of chronic hind limb ischemia to investigate therapeutic angiogenesis. Vascul Pharmacol. 2009. 51:268–274.25. Choi WI, Yoon KC, Im SK, Kim YH, Yuk SH, Tae G. Remarkably enhanced stability and function of core/shell nanoparticles composed of a lecithin core and a pluronic shell layer by photo-crosslinking the shell layer: in vitro and in vivo study. Acta Biomater. 2010. 6:2666–2673.26. Bussolati B, Mason JC. Dual role of VEGF-induced heme-oxygenase-1 in angiogenesis. Antioxid Redox Signal. 2006. 8:1153–1163.27. Ratner BD, Hoffman AS. Andrade JD, editor. Synthetic hydrogels for biomedical applications. Hydrogels for Medical and Related Applications. 1976. Washington: American Chemical Society;1–36. (ACS symposium series; vol 31).
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Angiogenesis Induced by Consecutive Intramuscular Injections of Vascular Endothelial Growth Factor in a Hindlimb Ischemic Mouse Model
- Angiogenic effects of recombinant thymosin beta4 in a mouse hindlimb ischemia model
- Weightlessness-simulated Experimental Apparatus: Hindlimb Unloading Model in Rat-Technical Aspects
- Melatonin-Induced PGC-1α Improves Angiogenic Potential of Mesenchymal Stem Cells in Hindlimb Ischemia
- Remote Ischemic Conditioning by Effluent Collected from a Novel Isolated Hindlimb Model Reduces Infarct Size in an Isolated Heart Model