Korean J Urol.
2006 Dec;47(12):1284-1288. 10.4111/kju.2006.47.12.1284.
Biological Variation of Serum Prostate Specific Antigen Levels in Men Aged 50 or Older without Prostate Cancer
- Affiliations
-
- 1Department of Urology, Hallym University College of Medicine, Chuncheon, Korea. js315@hallym.or.kr
- 2Department of Laboratory Medicine, Hallym University College of Medicine, Chuncheon, Korea.
- KMID: 2139772
- DOI: http://doi.org/10.4111/kju.2006.47.12.1284
Abstract
- PURPOSE
To evaluated the effect of the intra-individual variation in the serum prostate specific antigen (PSA) level in men without prostate cancer to decide on the requirement of a prostate biopsy.
MATERIALS AND METHODS
Patients, aged between 50 and 80 years, were screened for prostate cancer or lower urinary tract symptoms at least 2 times within 3 months using PSA or free PSA measurements. Patients with an initial PSA level between 2.0 and 10.0ng/ml were included. Those with prostate cancer, urinary tract infection or 5-alpha reductase inhibitor medication were excluded. The coefficient of variation (CV) was evaluated in each PSA range.
RESULTS
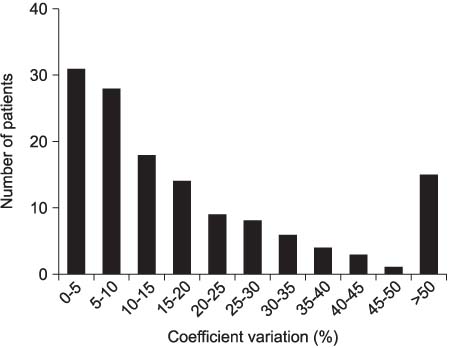
The analysis included 139 patients, with a mean age 62.1 years. The level of free PSA was measured in 56 patients. The mean interval between the two PSA measurements was 36.6 days. The mean CVs for the total PSA and % free PSA were 21.5 and 22.2%, respectively. 20% of patients show a CV of more than 30%, implying a large variation. In our study, the 95% confidence interval of initial PSA levels between 3.0 and 4.9ng/ml included the PSA cut-off point (4.0ng/ml) in the visit results.
CONCLUSIONS
The variation the PSA level was relatively small, but some patients showed a CV greater than 30%. Therefore, the intra-individual PSA variation should become part of interpreting the PSA test results, especially for men with a PSA value near the cut-off point.
MeSH Terms
Figure
Cited by 1 articles
-
Short-term Prostate-Specific Antigen Velocity Measurement before Prostate Biopsy
Jong Tak Park, Se Joong Kim, Hyun Soo Ahn, Young Soo Kim, Jong Bo Choi, Sun Il Kim
Korean J Urol. 2009;50(6):553-559. doi: 10.4111/kju.2009.50.6.553.
Reference
-
1. Roehrborn CG, Pickens GJ, Sanders JS. Diagnostic yield of repeated transrectal ultrasound-guided biopsies stratified by specific histopathologic diagnoses and prostate-specific antigen levels. Urology. 1996. 47:347–352.2. Carter HB, Pearson JD, Metter EJ, Brant LJ, Chan DW, Andres R, et al. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate diseases. JAMA. 1992. 267:2215–2220.3. Carter HB, Partin AW. Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Diagnosis and staging of prostate cancer. Campbell's urology. 2002. 8th ed. Philadelphia: Saunders;3055–3079.4. Prestigiacomo AF, Stamey TA. Physiological variation of serum prostate specific antigen in the 4.0 to 10.0ng/ml range in male volunteers. J Urol. 1996. 155:1977–1980.5. El-Shirbiny AM, Nilson T, Pawar HN. Serum prostate-specific antigen: hourly change/24 hours compared with prostatic acid phosphatase. Urology. 1990. 35:88–92.6. Dejter SW Jr, Martin JS, McPherson RA, Lynch JH. Daily variability in human serum prostate-specific antigen and prostatic acid phosphatase: a comparative evaluation. Urology. 1988. 32:288–292.7. Riehmann M, Rhodes PR, Cook TD, Grose GS, Bruskewitz RC. Analysis of variation in prostate-specific antigen values. Urology. 1993. 42:390–397.8. Roehrborn CG, Pickens GJ, Carmody T 3rd. Variability of repeated serum prostate-specific antigen (PSA) measurements within less than 90 days in a well-defined patient population. Urology. 1996. 47:59–66.9. Fleshner NE, O'Sullivan M, Fair WR. Prevalence and predictors of a positive repeat transrectal ultrasound guided needle biopsy of the prostate. J Urol. 1997. 158:505–508.10. Mian BM, Naya Y, Okihara K, Vakar-Lopez F, Torncoso P, Babaian RJ. Predictors of cancer in repeat extended multisite prostate biopsy in men with previous negative extended multisite biopsy. Urology. 2002. 60:836–840.11. Blijenberg BG, Lilja H, Neels H, Stenman UH. Quality assessment for prostate-specific antigen (PSA) in relation to ERSPC: report of the PSA committee. BJU Int. 2003. 92:Suppl 2. 66–70.12. Kim HS, Lim HW, Kim YL, Cho HS, Chun HS, Chun HS, et al. Annual report on external quality assessment of immunoassay subcommittee in Korea (2003). J Lab Med Qual Assuar. 2004. 26:103–121.13. Ornstein DK, Smith DS, Rao GS, Basler JW, Ratliff TL, Catalona WJ. Biological variation of total, free and percent free serum prostate specific antigen levels in screening volunteers. J Urol. 1997. 157:2179–2182.14. Morote J, Raventos CX, Lorente JA, Enbabo G, Lopez M, de Torres I. Intraindividual variations of total and percent free serum prostatic-specific antigen levels in patients with normal digital rectal examination. Eur Urol. 1999. 36:111–115.15. Komatsu K, Wehner N, Prestigiacomo AF, Chen Z, Stamey TA. Physiologic (intraindividual) variation of serum prostatespecific antigen in 814 men from a screening population. Urology. 1996. 47:343–346.16. Nixon RG, Lilly JD, Liedtke RJ, Batjer JD. Variation of free and total prostate-specific antigen levels: the effect on the percent free/total prostate-specific antigen. Arch Pathol Lab Med. 1997. 121:385–391.17. Tornblom M, Norming U, Becker C, Lilja H, Gustaffson O. Variation in percentage-free prostate-specific antigen (PSA) with prostate volume, age and total PSA level. BJU Int. 2001. 87:638–642.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Diagnostic Value of Prostate-specific Antigen and the of Routine Laboratory Examination for Early Detection
- The Factors Influencing the Percentage of Free Serum Prostate Specific Antigen Levels in Men without Clinically Detectable Prostate Cance
- Change of serum prostate specific antigen values after radiation therapy in prostate cancer
- Multiparametric MRI in the Detection of Clinically Significant Prostate Cancer
- The Age-specific Reference Ranges for Prostate-specific Antigen obtained from Health Promotion Center