Anat Cell Biol.
2012 Dec;45(4):259-267. 10.5115/acb.2012.45.4.259.
Dense distribution of macrophages in flexor aspects of the hand and foot of mid-term human fetuses
- Affiliations
-
- 1Department of Anatomy, Chonbuk National University Medical School, Jeonju, Korea.
- 2Oral Health Science Center hrc8, Tokyo Dental College, Chiba, Japan. abesh@tdc.ac.jp
- 3Department of Anatomy, Tokyo Dental College, Chiba, Japan.
- 4Maxillofacial Anatomy, Department of Maxillofacial Biology, Tokyo Medical and Dental University Graduate School, Tokyo, Japan.
- 5Division of Internal Medicine, Iwamizawa Kojin-kai Hospital, Iwamizawa, Japan.
- 6Department of Surgery, Chonbuk National University Medical School, Jeonju, Korea.
- KMID: 2133911
- DOI: http://doi.org/10.5115/acb.2012.45.4.259
Abstract
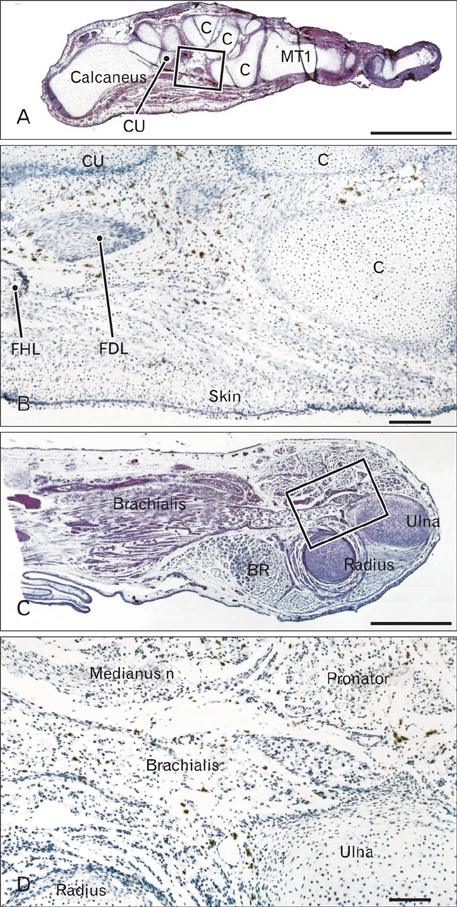
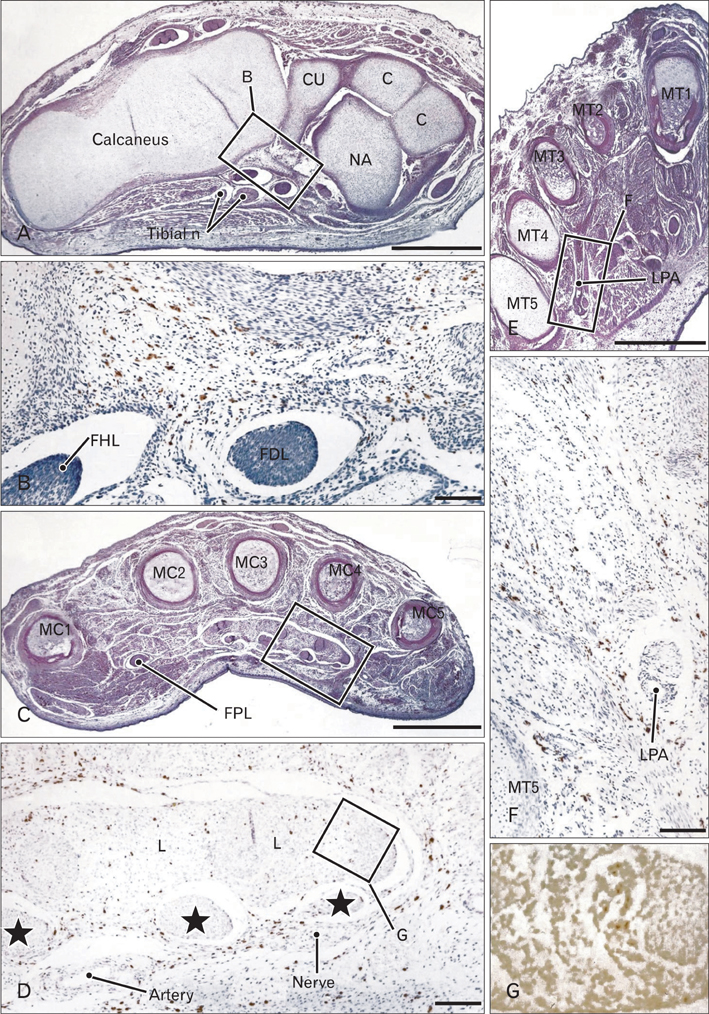
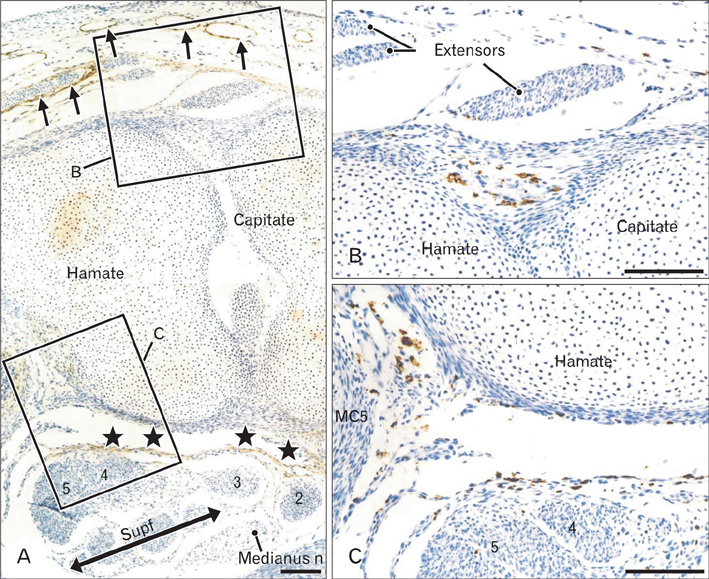
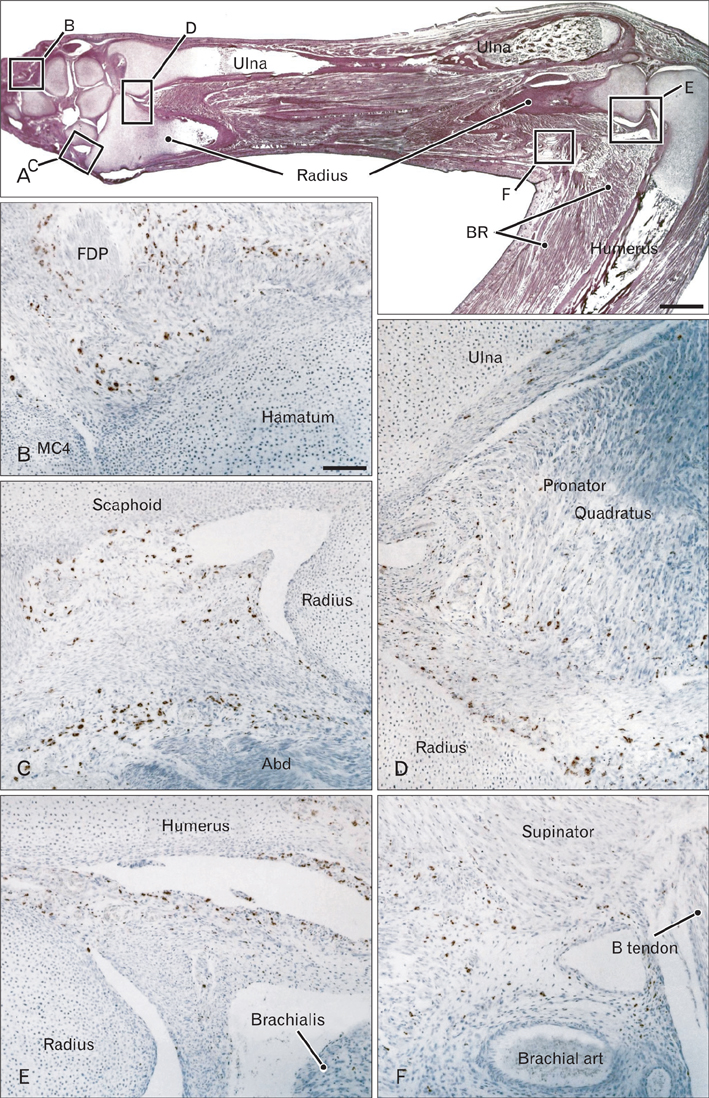
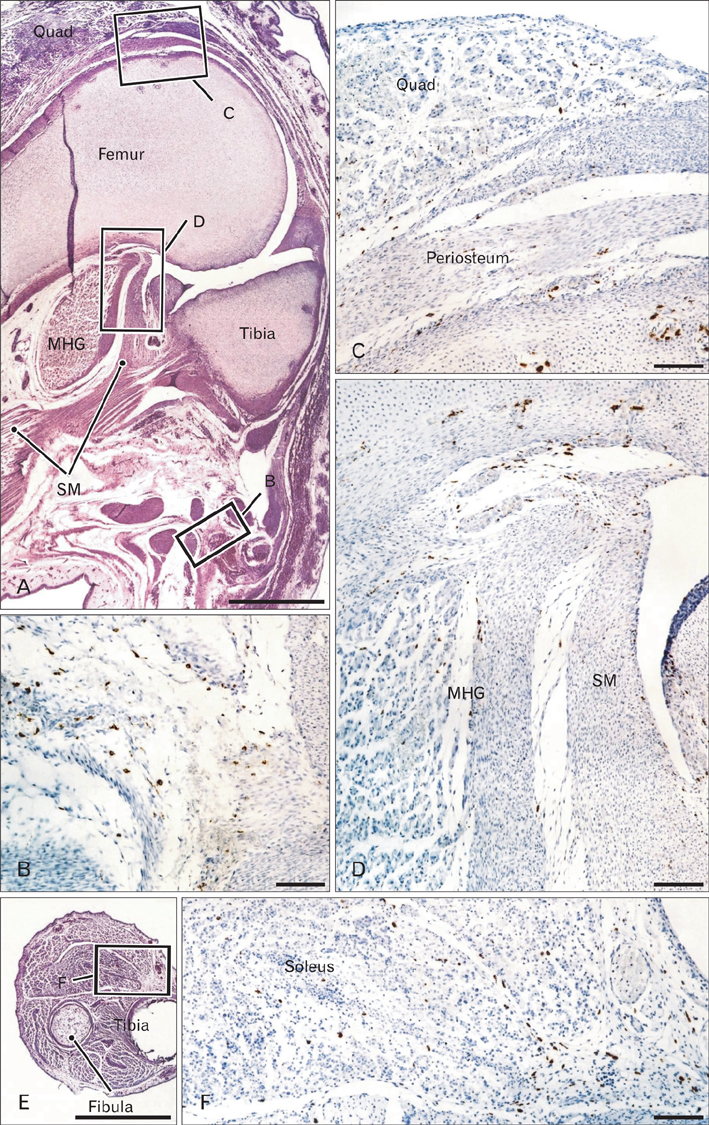
- In the developing human musculoskeletal system, cell death with macrophage accumulation occurs in the thigh muscle and interdigital area. To comprehensively clarify the distribution of macrophages, we immunohistochemically examined 16 pairs of upper and lower extremities without the hip joint (left and right sides) obtained from 8 human fetuses at approximately 10-15 weeks of gestation. Rather than in muscles, CD68-positive macrophages were densely distributed in loose connective tissues of the flexor aspects of the extremities, especially in the wrist, hand and foot. In contrast, no or fewer macrophages were evident in the shoulder and the extensor aspects of the extremities. The macrophages were not concentrated at the enthesis of the tendon and ligament, but tended to be arranged along other connective tissue fibers. Deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling revealed apoptosis in the hand lumbricalis muscles, but not in the area of macrophage accumulation. Likewise, podoplanin-positive lymphatic vessels were not localized to areas of macrophage accumulation. Re-organization of the connective tissue along and around the flexor tendons of the hand and foot, such as development of the bursa or tendon sheath at 10-15 weeks, might require the phagocytotic function of macrophages, although details of the mechanism remain unknown.
MeSH Terms
Figure
Reference
-
1. Benjamin M, McGonagle D. Basic concepts of enthesis biology and immunology. J Rheumatol Suppl. 2009. 83:12–13.2. Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. J Clin Invest. 2005. 115:2316–2319.3. Melrose J, Little CB. Immunolocalization of lymphatic vessels in human fetal knee joint tissues. Connect Tissue Res. 2010. 51:289–305.4. Ballard KJ, Holt SJ. Cytological and cytochemical studies on cell death and digestion in the foetal rat foot: the role of macrophages and hydrolytic enzymes. J Cell Sci. 1968. 3:245–262.5. Garcia-Martinez V, Macias D, Gañan Y, Garcia-Lobo JM, Francia MV, Fernandez-Teran MA, Hurle JM. Internucleosomal DNA fragmentation and programmed cell death (apoptosis) in the interdigital tissue of the embryonic chick leg bud. J Cell Sci. 1993. 106(Pt 1):201–208.6. Hopkinson-Woolley J, Hughes D, Gordon S, Martin P. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci. 1994. 107(Pt 5):1159–1167.7. Abood EA, Jones MM. Macrophages in developing mammalian skeletal muscle: evidence for muscle fibre death as a normal developmental event. Acta Anat (Basel). 1991. 140:201–212.8. Fidziańska A, Goebel HH. Human ontogenesis. 3. Cell death in fetal muscle. Acta Neuropathol. 1991. 81:572–577.9. McClearn D, Medville R, Noden D. Muscle cell death during the development of head and neck muscles in the chick embryo. Dev Dyn. 1995. 202:365–377.10. Schweichel JU, Merker HJ. The morphology of various types of cell death in prenatal tissues. Teratology. 1973. 7(3):253–266.11. Webb JN. The development of human skeletal muscle with particular reference to muscle cell death. J Pathol. 1972. 106:221–228.12. Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999. 154:385–394.13. Abe S, Rhee SK, Osonoi M, Nakamura T, Cho BH, Murakami G, Ide Y. Expression of intermediate filaments at muscle insertions in human fetuses. J Anat. 2010. 217:167–173.14. Sbernardori MC, Fenu G, Pirino A, Fabbriciani C, Montella A. Histogenesis and morphology of the flexor tendon pulley system in the human embryonic hand. J Hand Surg Br. 2000. 25:175–179.15. Rowshan K, Hadley S, Pham K, Caiozzo V, Lee TQ, Gupta R. Development of fatty atrophy after neurologic and rotator cuff injuries in an animal model of rotator cuff pathology. J Bone Joint Surg Am. 2010. 92:2270–2278.16. Takahashi M, Ward SR, Marchuk LL, Frank CB, Lieber RL. Asynchronous muscle and tendon adaptation after surgical tensioning procedures. J Bone Joint Surg Am. 2010. 92:664–674.17. Cho KH, Kim JH, Ha YS, Murakami G, Cho BH, Abe S. Development of the deep flexor tendons and lumbricalis muscle in the hand and foot: a histological study using human mid-term foetuses. Folia Morphol (Warsz). 2012. 71:154–163.18. Castro-Obregón S, Del Rio G, Chen SF, Swanson RA, Frankowski H, Rao RV, Stoka V, Vesce S, Nicholls DG, Bredesen DE. A ligand-receptor pair that triggers a non-apoptotic form of programmed cell death. Cell Death Differ. 2002. 9:807–817.19. Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl). 1990. 181:195–213.20. Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995. 146:3–15.21. Jin ZW, Nakamura T, Yu HC, Kimura W, Murakami G, Cho BH. Fetal anatomy of peripheral lymphatic vessels: a D2-40 immunohistochemical study using an 18-week human fetus (CRL 155 mm). J Anat. 2010. 216:671–682.22. Jin ZW, Song KJ, Lee NH, Nakamura T, Fujimiya M, Murakami G, Cho BH. Contribution of the anterior longitudinal ligament to ossification and growth of the vertebral body: an immunohistochemical study using the human fetal lumbar vertebrae. Surg Radiol Anat. 2011. 33:11–18.23. Abe S, Suzuki M, Cho KH, Murakami G, Cho BH, Ide Y. CD34-positive developing vessels and other structures in human fetuses: an immunohistochemical study. Surg Radiol Anat. 2011. 33:919–927.24. Kjaer M, Magnusson P, Krogsgaard M, Boysen Møller J, Olesen J, Heinemeier K, Hansen M, Haraldsson B, Koskinen S, Esmarck B, Langberg H. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat. 2006. 208:445–450.25. Okita M, Yoshimura T, Nakano J, Motomura M, Eguchi K. Effects of reduced joint mobility on sarcomere length, collagen fibril arrangement in the endomysium, and hyaluronan in rat soleus muscle. J Muscle Res Cell Motil. 2004. 25:159–166.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fibroma of the Extensor Digitorum Longus and Extensor Digitorum Brevis Conjoined Tendon Sheath: A Case Report
- A temporary disc-like structure at the median atlanto-axial joint in human fetuses
- Removal of a Movable Foreign Body in the Flexor Tendon Sheath of the Hand: A Case Report
- Isolation of Echovirus Serotype 25 from Patient with Hand , Foot and Mouth Disease in Pusan , 1998
- The Clinical Comparison of Ganglions in Hand and Foot