Cancer Res Treat.
2015 Apr;47(2):182-188. 10.4143/crt.2013.227.
The Impact of Molecularly Targeted Treatment on Direct Medical Costs in Patients with Advanced Non-small Cell Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. kimdw@snu.ac.kr
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Outcomes Research/Evidence Based Medicine Team, Pfizer Pharmaceuticals Korea Ltd., Seoul, Korea.
- KMID: 2132796
- DOI: http://doi.org/10.4143/crt.2013.227
Abstract
- PURPOSE
To investigate the impact of targeted treatment on direct medical costs of patients with advanced non-small cell lung cancer (NSCLC).
MATERIALS AND METHODS
Medical records of 108 stage IIIB/IV NSCLC patients treated in Seoul National University Hospital between 2003 and 2009, were reviewed to collect medical resources utilization data from the diagnosis of stage IIIB/IV NSCLC to the end of active anti-cancer treatment. The direct medical costs were calculated by multiplying the number of medical resources used by the unit price. All costs were expressed in US dollars for each patient.
RESULTS
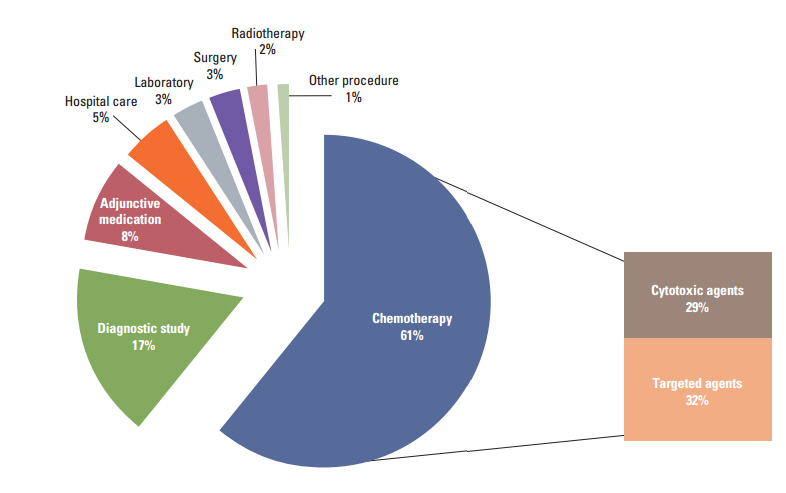
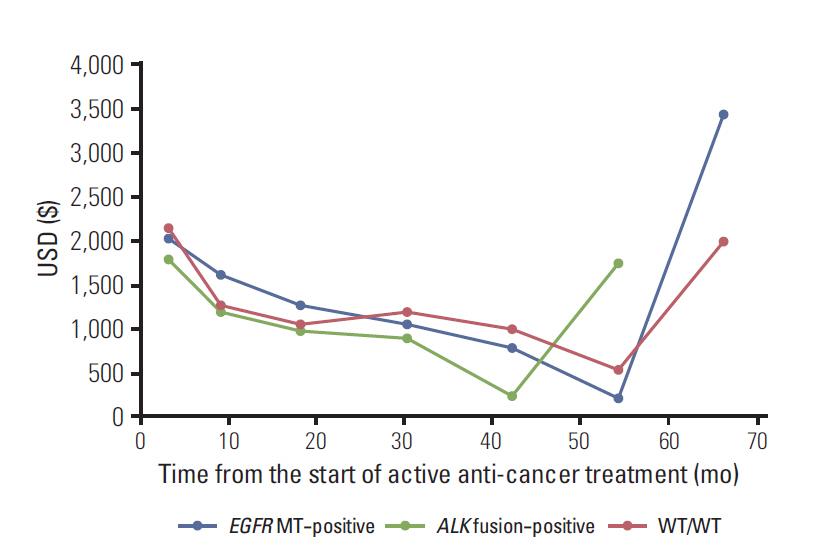
The mean total direct medical costs were $34,732 (standard deviation, 21,168) in the study cohort. The mean total direct medical costs were higher in epidermal growth factor receptor (EGFR) mutation (EGFR MT)-positive patients than EGFR wild-type (EGFR WT) patients ($41,403 vs. $30,146, p=0.005). However, the mean monthly direct medical costs did not differ significantly between EGFR MT-positive patients and EGFR WT patients ($2,120 vs. $2,702, p=0.119) because of the longer duration of active anti-cancer treatment in EGFR MT-positive patients. This discrepancy was mainly attributable to EGFR MT-positive patients' lower non-chemotherapy costs ($948 vs. $1,522, p=0.007). The total and monthly direct medical costs of ALK fusion-positive patients who did not receive ALK inhibitors did not differ from WT/WT patients.
CONCLUSION
This study suggests that the availability of targeted agents for EGFR MT-positive patients lowers the mean monthly medical costs by prolonging survival and diminishing the use of other medical resources, despite the considerable drug costs.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
Article2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29.
Article3. Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008; 100:630–41.
Article4. Ettinger DS, Akerley W, Bepler G, Blum MG, Chang A, Cheney RT, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2010; 8:740–801.5. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–57.
Article6. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010; 362:2380–8.
Article7. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010; 11:121–8.
Article8. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as firstline treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13:239–46.9. Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013; 368:2385–94.
Article10. Bongers ML, Coupe VM, Jansma EP, Smit EF, Uyl-de Groot CA. Cost effectiveness of treatment with new agents in advanced non-small-cell lung cancer: a systematic review. Pharmacoeconomics. 2012; 30:17–34.11. Cromwell I, van der Hoek K, Malfair Taylor SC, Melosky B, Peacock S. Erlotinib or best supportive care for third-line treatment of advanced non-small-cell lung cancer: a real-world cost-effectiveness analysis. Lung Cancer. 2012; 76:472–7.
Article12. Vergnenegre A, Ray JA, Chouaid C, Grossi F, Bischoff HG, Heigener DF, et al. Cross-market cost-effectiveness analysis of erlotinib as first-line maintenance treatment for patients with stable non-small cell lung cancer. Clinicoecon Outcomes Res. 2012; 4:31–7.13. Chouaid C, Le Caer H, Corre R, Crequit J, Locher C, Falchero L, et al. Cost analysis of erlotinib versus chemotherapy for first-line treatment of non-small-cell lung cancer in frail elderly patients participating in a prospective phase 2 study (GFPC 0505). Clin Lung Cancer. 2013; 14:103–7.
Article14. Walleser S, Ray J, Bischoff H, Vergnenegre A, Rosery H, Chouaid C, et al. Maintenance erlotinib in advanced nonsmall cell lung cancer: cost-effectiveness in EGFR wild-type across Europe. Clinicoecon Outcomes Res. 2012; 4:269–75.15. Lee JK, Park HS, Kim DW, Kulig K, Kim TM, Lee SH, et al. Comparative analyses of overall survival in patients with anaplastic lymphoma kinase-positive and matched wild-type advanced nonsmall cell lung cancer. Cancer. 2012; 118:3579–86.
Article16. Gadgeel SM, Cote ML, Schwartz AG, Matherly LH, Wozniak A, Bepler G. Parameters for individualizing systemic therapy in non-small cell lung cancer. Drug Resist Updat. 2010; 13:196–204.
Article17. Horgan AM, Bradbury PA, Amir E, Ng R, Douillard JY, Kim ES, et al. An economic analysis of the INTEREST trial, a randomized trial of docetaxel versus gefitinib as second-/third-line therapy in advanced non-small-cell lung cancer. Ann Oncol. 2011; 22:1805–11.
Article18. Bouwes N. The cost of illness handbook [Internet]. Washington, DC: U.S. Environmental Protection Agency; 2010 [cited 2014 Jan 21]. Available from: http://www.epa.gov/oppt/coi/pubs/toc.html.19. McDuffie HH, Klaassen DJ, Dosman JA. Female-male differences in patients with primary lung cancer. Cancer. 1987; 59:1825–30.
Article20. de Perrot M, Licker M, Bouchardy C, Usel M, Robert J, Spiliopoulos A. Sex differences in presentation, management, and prognosis of patients with non-small cell lung carcinoma. J Thorac Cardiovasc Surg. 2000; 119:21–6.
Article21. Emanuel EJ, Ash A, Yu W, Gazelle G, Levinsky NG, Saynina O, et al. Managed care, hospice use, site of death, and medical expenditures in the last year of life. Arch Intern Med. 2002; 162:1722–8.
Article22. Zhang B, Wright AA, Huskamp HA, Nilsson ME, Maciejewski ML, Earle CC, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009; 169:480–8.