J Gynecol Oncol.
2014 Jul;25(3):249-259. 10.3802/jgo.2014.25.3.249.
Beyond angiogenesis blockade: targeted therapy for advanced cervical cancer
- Affiliations
-
- 1Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of California Irvine Medical Center, Orange, CA, USA. ktewari@uci.edu
- KMID: 2130106
- DOI: http://doi.org/10.3802/jgo.2014.25.3.249
Abstract
- The global burden of advanced stage cervical cancer remains significant, particular in resource poor countries where effective screening programs are absent. Unfortunately, a proportion of patients will be diagnosed with advanced stage disease, and may suffer from persistent or recurrent disease despite treatment with combination chemotherapy and radiation. Patients with recurrent disease have a poor salvage rate, with an expected 5-year survival of less than 10%. Recently, significant gains have been made in the antiangiogenic arena; nonetheless the need to develop effective alternate targeted strategies is implicit. As such, a review of molecular targeted therapy in the treatment of this disease is warranted. In an era of biologics, combined therapy with cytotoxic drugs and molecular targeted agents, represents an exciting arena yet to be fully explored.
MeSH Terms
-
Angiogenesis Inhibitors/therapeutic use
Antineoplastic Agents/*therapeutic use
Female
Histone Deacetylase Inhibitors/therapeutic use
Humans
Molecular Targeted Therapy/*methods
Receptor, Epidermal Growth Factor/antagonists & inhibitors
Salvage Therapy/methods
Uterine Cervical Neoplasms/*drug therapy
Angiogenesis Inhibitors
Antineoplastic Agents
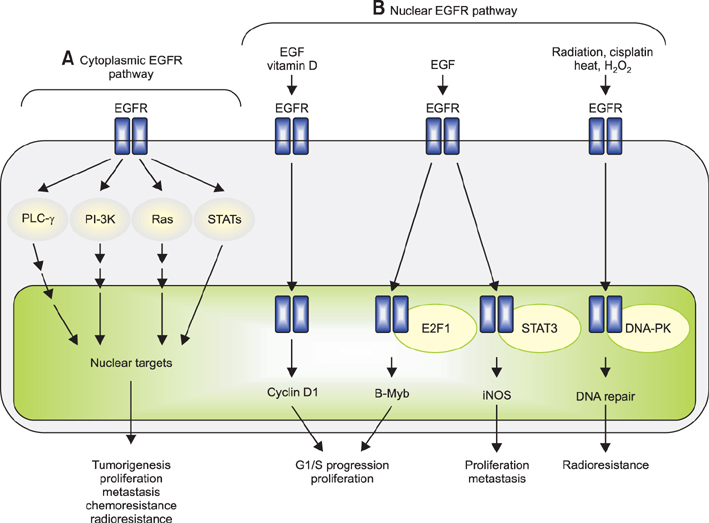
Histone Deacetylase Inhibitors
Receptor, Epidermal Growth Factor
Figure
Reference
-
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64:9–29.3. Diaz-Padilla I, Monk BJ, Mackay HJ, Oaknin A. Treatment of metastatic cervical cancer: future directions involving targeted agents. Crit Rev Oncol Hematol. 2013; 85:303–314.4. Greer BE, Koh WJ, Abu-Rustum NR, Apte SM, Campos SM, Chan J, et al. Cervical cancer. J Natl Compr Canc Netw. 2010; 8:1388–1416.5. Thigpen T, Shingleton H, Homesley H, Lagasse L, Blessing J. Cis-platinum in treatment of advanced or recurrent squamous cell carcinoma of the cervix: a phase II study of the Gynecologic Oncology Group. Cancer. 1981; 48:899–903.6. McGuire WP 3rd, Arseneau J, Blessing JA, DiSaia PJ, Hatch KD, Given FT Jr, et al. A randomized comparative trial of carboplatin and iproplatin in advanced squamous carcinoma of the uterine cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1989; 7:1462–1468.7. Fracasso PM, Blessing JA, Wolf J, Rocereto TF, Berek JS, Waggoner S. Phase II evaluation of oxaliplatin in previously treated squamous cell carcinoma of the cervix: a gynecologic oncology group study. Gynecol Oncol. 2003; 90:177–180.8. Thigpen T, Blessing JA, Gallup DG, Maiman M, Soper JT. Phase II trial of mitomycin-C in squamous cell carcinoma of the uterine cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1995; 57:376–379.9. Sutton GP, Blessing JA, Adcock L, Webster KD, DeEulis T. Phase II study of ifosfamide and mesna in patients with previously-treated carcinoma of the cervix: a Gynecologic Oncology Group study. Invest New Drugs. 1989; 7:341–343.10. Look KY, Blessing JA, Levenback C, Kohler M, Chafe W, Roman LD. A phase II trial of CPT-11 in recurrent squamous carcinoma of the cervix: a gynecologic oncology group study. Gynecol Oncol. 1998; 70:334–338.11. Schilder RJ, Blessing JA, Morgan M, Mangan CE, Rader JS. Evaluation of gemcitabine in patients with squamous cell carcinoma of the cervix: a Phase II study of the gynecologic oncology group. Gynecol Oncol. 2000; 76:204–207.12. Bookman MA, Blessing JA, Hanjani P, Herzog TJ, Andersen WA. Topotecan in squamous cell carcinoma of the cervix: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2000; 77:446–449.13. Curtin JP, Blessing JA, Webster KD, Rose PG, Mayer AR, Fowler WC Jr, et al. Paclitaxel, an active agent in nonsquamous carcinomas of the uterine cervix: a Gynecologic Oncology Group Study. J Clin Oncol. 2001; 19:1275–1278.14. McGuire WP, Blessing JA, Moore D, Lentz SS, Photopulos G. Paclitaxel has moderate activity in squamous cervix cancer: a Gynecologic Oncology Group study. J Clin Oncol. 1996; 14:792–795.15. Garcia AA, Blessing JA, Vaccarello L, Roman LD. Gynecologic Oncology Group Study. Phase II clinical trial of docetaxel in refractory squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. Am J Clin Oncol. 2007; 30:428–431.16. Muggia FM, Blessing JA, Waggoner S, Berek JS, Monk BJ, Sorosky J, et al. Evaluation of vinorelbine in persistent or recurrent nonsquamous carcinoma of the cervix: a Gynecologic Oncology Group Study. Gynecol Oncol. 2005; 96:108–111.17. Bloss JD, Blessing JA, Behrens BC, Mannel RS, Rader JS, Sood AK, et al. Randomized trial of cisplatin and ifosfamide with or without bleomycin in squamous carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2002; 20:1832–1837.18. Long HJ 3rd, Bundy BN, Grendys EC Jr, Benda JA, McMeekin DS, Sorosky J, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group Study. J Clin Oncol. 2005; 23:4626–4633.19. Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009; 27:4649–4655.20. Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2004; 22:3113–3119.21. Omura GA, Blessing JA, Vaccarello L, Berman ML, Clarke-Pearson DL, Mutch DG, et al. Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1997; 15:165–171.22. Tewari KS, Sill MW, Long HJ 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014; 370:734–743.23. Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer. 2006; 94:184–188.24. Milas L, Mason K, Hunter N, Petersen S, Yamakawa M, Ang K, et al. In vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibody. Clin Cancer Res. 2000; 6:701–708.25. Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000; 6:2166–2174.26. Bianco C, Bianco R, Tortora G, Damiano V, Guerrieri P, Montemaggi P, et al. Antitumor activity of combined treatment of human cancer cells with ionizing radiation and anti-epidermal growth factor receptor monoclonal antibody C225 plus type I protein kinase A antisense oligonucleotide. Clin Cancer Res. 2000; 6:4343–4350.27. Akimoto T, Hunter NR, Buchmiller L, Mason K, Ang KK, Milas L. Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas. Clin Cancer Res. 1999; 5:2884–2890.28. Zagouri F, Sergentanis TN, Chrysikos D, Filipits M, Bartsch R. Molecularly targeted therapies in cervical cancer: a systematic review. Gynecol Oncol. 2012; 126:291–303.29. del Campo JM, Prat A, Gil-Moreno A, Perez J, Parera M. Update on novel therapeutic agents for cervical cancer. Gynecol Oncol. 2008; 110(3):Suppl 2. S72–S76.30. Santin AD, Sill MW, McMeekin DS, Leitao MM Jr, Brown J, Sutton GP, et al. Phase II trial of cetuximab in the treatment of persistent or recurrent squamous or non-squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 2011; 122:495–500.31. Hertlein L, Lenhard M, Kirschenhofer A, Kahlert S, Mayr D, Burges A, et al. Cetuximab monotherapy in advanced cervical cancer: a retrospective study with five patients. Arch Gynecol Obstet. 2011; 283:109–113.32. Farley J, Sill MW, Birrer M, Walker J, Schilder RJ, Thigpen JT, et al. Phase II study of cisplatin plus cetuximab in advanced, recurrent, and previously treated cancers of the cervix and evaluation of epidermal growth factor receptor immunohistochemical expression: a Gynecologic Oncology Group study. Gynecol Oncol. 2011; 121:303–308.33. Kurtz JE, Hardy-Bessard AC, Deslandres M, Lavau-Denes S, Largillier R, Roemer-Becuwe C, et al. Cetuximab, topotecan and cisplatin for the treatment of advanced cervical cancer: a phase II GINECO trial. Gynecol Oncol. 2009; 113:16–20.34. Goncalves A, Fabbro M, Lhomme C, Gladieff L, Extra JM, Floquet A, et al. A phase II trial to evaluate gefitinib as second- or third-line treatment in patients with recurring locoregionally advanced or metastatic cervical cancer. Gynecol Oncol. 2008; 108:42–46.35. Nogueira-Rodrigues A, do Carmo CC, Viegas C, Erlich F, Camisao C, Fontao K, et al. Phase I trial of erlotinib combined with cisplatin and radiotherapy for patients with locally advanced cervical squamous cell cancer. Clin Cancer Res. 2008; 14:6324–6329.36. Schilder RJ, Sill MW, Lee YC, Mannel R. A phase II trial of erlotinib in recurrent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. Int J Gynecol Cancer. 2009; 19:929–933.37. Monk BJ, Mas Lopez L, Zarba JJ, Oaknin A, Tarpin C, Termrungruanglert W, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J Clin Oncol. 2010; 28:3562–3569.38. Lee CM, Shrieve DC, Zempolich KA, Lee RJ, Hammond E, Handrahan DL, et al. Correlation between human epidermal growth factor receptor family (EGFR, HER2, HER3, HER4), phosphorylated Akt (P-Akt), and clinical outcomes after radiation therapy in carcinoma of the cervix. Gynecol Oncol. 2005; 99:415–421.39. Takai N, Kira N, Ishii T, Nishida M, Nasu K, Narahara H. Novel chemotherapy using histone deacetylase inhibitors in cervical cancer. Asian Pac J Cancer Prev. 2011; 12:575–580.40. Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002; 1:287–299.41. Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006; 6:38–51.42. Atmaca A, Al-Batran SE, Maurer A, Neumann A, Heinzel T, Hentsch B, et al. Valproic acid (VPA) in patients with refractory advanced cancer: a dose escalating phase I clinical trial. Br J Cancer. 2007; 97:177–182.43. Chavez-Blanco A, Segura-Pacheco B, Perez-Cardenas E, Taja-Chayeb L, Cetina L, Candelaria M, et al. Histone acetylation and histone deacetylase activity of magnesium valproate in tumor and peripheral blood of patients with cervical cancer: a phase I study. Mol Cancer. 2005; 4:22.44. Coronel J, Cetina L, Pacheco I, Trejo-Becerril C, Gonzalez-Fierro A, de la Cruz-Hernandez E, et al. A double-blind, placebo-controlled, randomized phase III trial of chemotherapy plus epigenetic therapy with hydralazine valproate for advanced cervical cancer: preliminary results. Med Oncol. 2011; 28:Suppl 1. S540–S546.45. Candelaria M, Cetina L, Perez-Cardenas E, de la Cruz-Hernandez E, Gonzalez-Fierro A, Trejo-Becerril C, et al. Epigenetic therapy and cisplatin chemoradiation in FIGO stage IIIB cervical cancer. Eur J Gynaecol Oncol. 2010; 31:386–391.46. Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002; 2:489–501.47. Diaz-Padilla I, Duran I, Clarke BA, Oza AM. Biologic rationale and clinical activity of mTOR inhibitors in gynecological cancer. Cancer Treat Rev. 2012; 38:767–775.48. Lee DF, Hung MC. All roads lead to mTOR: integrating inflammation and tumor angiogenesis. Cell Cycle. 2007; 6:3011–3014.49. Liu D, Hou P, Liu Z, Wu G, Xing M. Genetic alterations in the phosphoinositide 3-kinase/Akt signaling pathway confer sensitivity of thyroid cancer cells to therapeutic targeting of Akt and mammalian target of rapamycin. Cancer Res. 2009; 69:7311–7319.50. Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009; 8:627–644.51. Hirai H, Arai T, Okada M, Nishibata T, Kobayashi M, Sakai N, et al. MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor efficacy of various DNA-damaging agents, including 5-fluorouracil. Cancer Biol Ther. 2010; 9:514–522.52. Dixon H, Norbury CJ. Therapeutic exploitation of checkpoint defects in cancer cells lacking p53 function. Cell Cycle. 2002; 1:362–368.53. Wang Y, Decker SJ, Sebolt-Leopold J. Knockdown of Chk1, Wee1 and Myt1 by RNA interference abrogates G2 checkpoint and induces apoptosis. Cancer Biol Ther. 2004; 3:305–313.54. Hu YY, Zheng MH, Zhang R, Liang YM, Han H. Notch signaling pathway and cancer metastasis. Adv Exp Med Biol. 2012; 727:186–198.55. Guo S, Liu M, Gonzalez-Perez RR. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim Biophys Acta. 2011; 1815:197–213.56. Borggrefe T, Oswald F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci. 2009; 66:1631–1646.57. Lewis J. Notch signalling and the control of cell fate choices in vertebrates. Semin Cell Dev Biol. 1998; 9:583–589.58. Lewis J. Notch signalling: a short cut to the nucleus. Nature. 1998; 393:304–305.59. Simpson P. Developmental genetics: the Notch connection. Nature. 1995; 375:736–737.60. Daniel B, Rangarajan A, Mukherjee G, Vallikad E, Krishna S. The link between integration and expression of human papillomavirus type 16 genomes and cellular changes in the evolution of cervical intraepithelial neoplastic lesions. J Gen Virol. 1997; 78:1095–1101.61. Zagouras P, Stifani S, Blaumueller CM, Carcangiu ML, Artavanis-Tsakonas S. Alterations in Notch signaling in neoplastic lesions of the human cervix. Proc Natl Acad Sci U S A. 1995; 92:6414–6418.62. Maliekal TT, Bajaj J, Giri V, Subramanyam D, Krishna S. The role of Notch signaling in human cervical cancer: implications for solid tumors. Oncogene. 2008; 27:5110–5114.63. Koga F, Kihara K, Neckers L. Inhibition of cancer invasion and metastasis by targeting the molecular chaperone heat-shock protein 90. Anticancer Res. 2009; 29:797–807.64. Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005; 10:86–103.65. Hwang YJ, Lee SP, Kim SY, Choi YH, Kim MJ, Lee CH, et al. Expression of heat shock protein 60 kDa is upregulated in cervical cancer. Yonsei Med J. 2009; 50:399–406.66. Bohonowych JE, Gopal U, Isaacs JS. Hsp90 as a gatekeeper of tumor angiogenesis: clinical promise and potential pitfalls. J Oncol. 2010; 2010:412985.67. Abd el All H, Rey A, Duvillard P. Expression of heat shock protein 70 and c-myc in cervical carcinoma. Anticancer Res. 1998; 18(3A):1533–1536.68. Bisht KS, Bradbury CM, Mattson D, Kaushal A, Sowers A, Markovina S, et al. Geldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicity. Cancer Res. 2003; 63:8984–8995.69. Reinbolt RE, Hays JL. The role of PARP inhibitors in the treatment of gynecologic malignancies. Front Oncol. 2013; 3:237.70. Saijo N. Present status and problems on molecular targeted therapy of cancer. Cancer Res Treat. 2012; 44:1–10.71. Michels J, Vitale I, Galluzzi L, Adam J, Olaussen KA, Kepp O, et al. Cisplatin resistance associated with PARP hyperactivation. Cancer Res. 2013; 73:2271–2280.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Targeted therapy and tailored chemotherapy for advanced non-small cell lung cancer
- Clinical Value of Serum Vascular Endothelial Growth Factor ( VEGF ) in Cervical Cancer Patients
- Angiogenesis in Cervical Intraepithelial Neoplasia and Invasive Cervical Cancer: an Immunohistochemical Study
- Serum and Urine basic Fibroblast Growth Factor (bFGF) in Cervical Cancer Patients
- Systemic Therapy for Advanced Hepatocellular Carcinoma: Targeted Therapy and Immunotherapy