J Korean Ophthalmol Soc.
2008 Feb;49(2):282-287. 10.3341/jkos.2008.49.2.282.
Measurement and Analysis of Serous Fluid in Central Serous Chorioretinopathy using OCT
- Affiliations
-
- 1Department of Ophthalmology, College of Medicine, Inje University, Sanggye Paik Hospital, Seoul, Korea. eyedoctor@freechal.com
- 2Department of Ophthalmology, College of Medicine, Inje University, Seoul Paik Hospital, Seoul, Korea.
- KMID: 2127120
- DOI: http://doi.org/10.3341/jkos.2008.49.2.282
Abstract
-
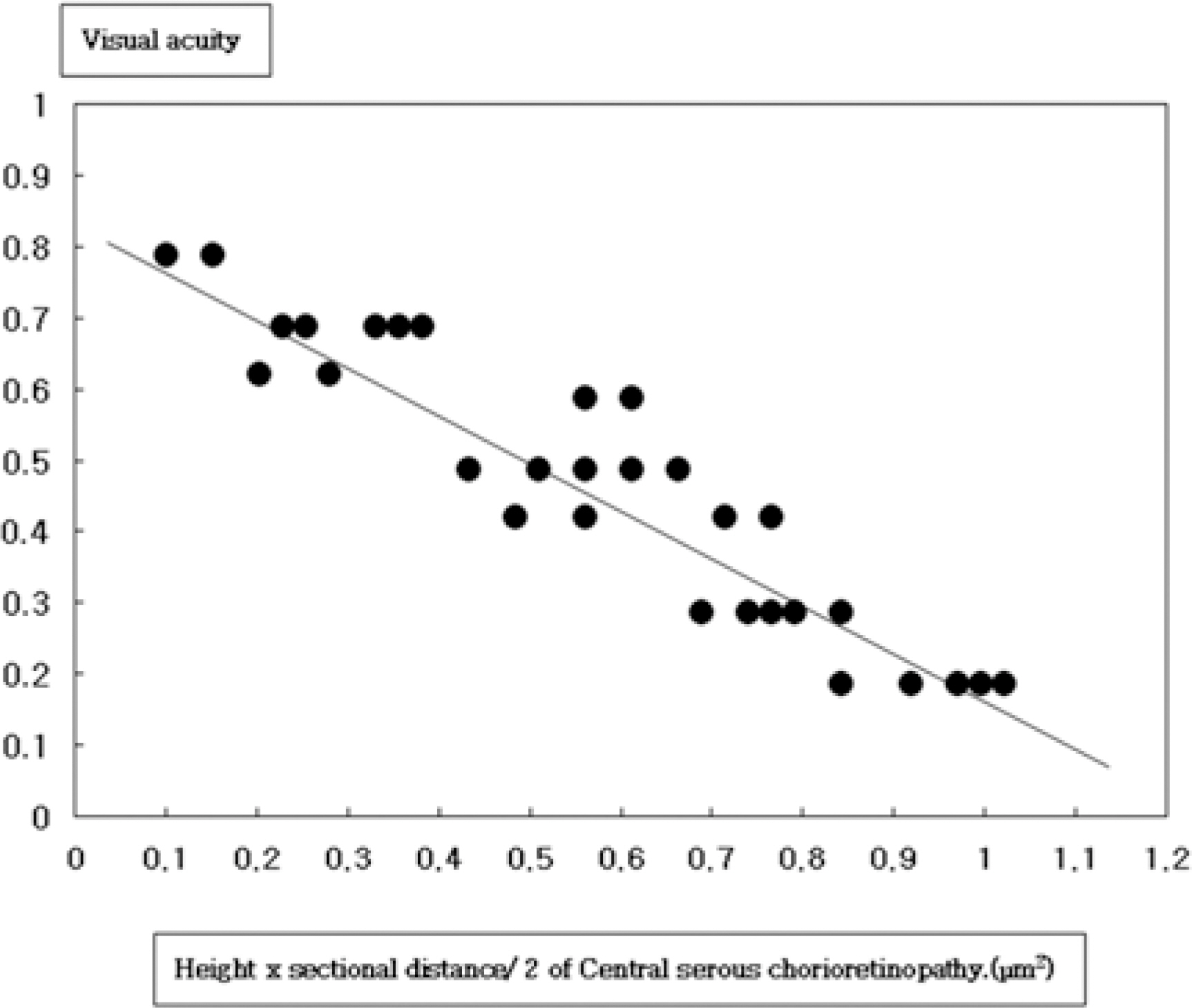
PURPOSE: To evaluate the correlation between the degree of serous fluid and best corrected visual acuity at the first visit in central serous chorioretinopathy using optical coherence tomography.
METHODS
Retrospective analysis was performed for 30 eyes of 30 patients with central serous chorioretinopathy. Cross-sectional retinal images through the center of the fovea were obtained from all eyes by optical coherence tomography. The height, sectional area, and the tangent of theta (tan theta) were estimated. They were statistically analyzed and correlated with best corrected visual acuity.
RESULTS
Mean (+/-SD) height of serous fluid was 341.40+/-120.60 micrometer, mean (+/-SD) sectional area was 0.55+/-0.29 mm2, and mean (+/-SD) tan theta was 0.22+/-0.04. The correlation coefficients between best corrected visual acuity at the first visit and sectional area were r=-0.740 (P=0.001).
CONCLUSIONS
Optical coherence tomography is useful for the quantitative evaluation of serous neurosensory retinal detachment and estimating visual acuity in central serous chorioretinopathy.
MeSH Terms
Figure
Reference
-
References
1. Gass JD. Pathogenesis of disciform detachment of the neuro-epithelium. II. Idiopathic central serous chorioretinopathy. Am J Ophthalmol. 1967; 63:1–139.2. Yannuzzi LA, Gitter KA, Schatz H. The macula: a comprehensive text and atlas. 2nd ed.Baltimore: William&Wilkins;1982. p. 145–65.3. Ie D, Yannuzzi LA, Spaide RF, et al. Subretinal exudative deposits in central serous chorioretinopathy. Br J Ophthalmol. 1991; 109:677–81.
Article4. Ueoka T. A pathologic study of central serous chorioretinopathy. Acta Soc Ophthalmol Jpn. 1949; 53:222–6.5. Bennett G. Central serous retinopathy. Br J Ophthalmol. 1995; 39:605–18.
Article6. Iida T, Hagimura N, Sato T, Kishi S. Evaluation of central serous chorioretinopathy with optical coherence tomography. Am J Ophthalmol. 2000; 129:16–20.
Article7. Hee MR, Puliafito CA, Wong C, et al. Optical coherence tomography of central serous chorioretinopathy. Am J Ophthalmol. 1995; 120:65–74.
Article8. Iida T, Yannuzzi LA, Spaide RF, et al. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003; 23:1–7.
Article9. Wang MS, Sander B, Larsen M. Retinal atrophy in idiopathic central serous chorioretinopathy. Am J Ophthalmol. 2002; 133:787–93.10. Negi A, Marmor MF. The resorption of subretinal fluid after diffuse damage of th retinal pigment damage. Invest Ophthalmol Vis Sci. 1983; 24:1475–9.11. Guyer DR, Yannuzzi LA, Slakter JS, et al. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994; 112:1057–62.
Article12. De Venecia G. Fluorescein angiographic smoke stack. Case presention at Verhoeff Society Meeting. Washington DC. 1982. 24–5.13. Stamer WD, Bok D, Hu J, et al. Aquaporin-1 channels in human retinal pigment epithelium: Role in transepithelial water movement. Invest Ophthalmol Vis Sci. 2003; 44:2803–8.
Article14. Fine BS, Brucker AJ. Macular edema and cystoid macular edema. Am J Ophthalmol. 1995; 120:65–74.
Article15. Yanoff M, Fine BS, Brucker AJ, Eagle RC. Pathology of human cystoid macular edema. Surv Ophthalmol. 1984; 28:505–11.
Article16. Loo RH, Scott IU, Flynn HW, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002; 22:19–24.
Article17. Sakai T, Calderone JB, Lewis GP, et al. Cone photoreceptor recovery after experimental detachment and reattachment: an immunocytochemical, morphological, and electrophysiological study. Invest Ophthalmol Vis Sci. 2003; 44:416–25.
Article18. Bujarborua D. Long-term follow-up of idiopathic central serous chorioretinopathy without laser. Acta Ophthalmol Scand. 2001; 79:417–21.
Article19. Piccolino FC, De La Longrais RR, Ravera G, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005; 139:87–99.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Use fulness of OCT[Optical Coherence Tomography]for the Diagnosis of Central Serous Choriore tinopathy
- Central Serous Chorioretinopathy in a Patient with Retinal Macrovessel
- A case of Atypical Central Serous Chorioretinopathy with Bullous Retinal Detachment
- Stellate Ganglion Block for Treatment of Central Serous Chorioretinopathy
- A Case of Central Serous Chorioretinopathy Following Systemic Corticosteroid Therapy