J Adv Prosthodont.
2013 Aug;5(3):341-350. 10.4047/jap.2013.5.3.341.
Focal adhesion linker proteins expression of fibroblast related to adhesion in response to different transmucosal abutment surfaces
- Affiliations
-
- 1Dental Science Research Institute, School of Dentistry, Chonnam National University, Gwangju, Republic of Korea. greatone@chonnam.ac.kr
- KMID: 2118175
- DOI: http://doi.org/10.4047/jap.2013.5.3.341
Abstract
- PURPOSE
To evaluate adherence of human gingival fibroblasts (HGFs) to transmucosal abutment of dental implant with different surface conditions with time and to investigate the roles of focal adhesion linker proteins (FALPs) involved in HGFs adhesion to abutment surfaces.
MATERIALS AND METHODS
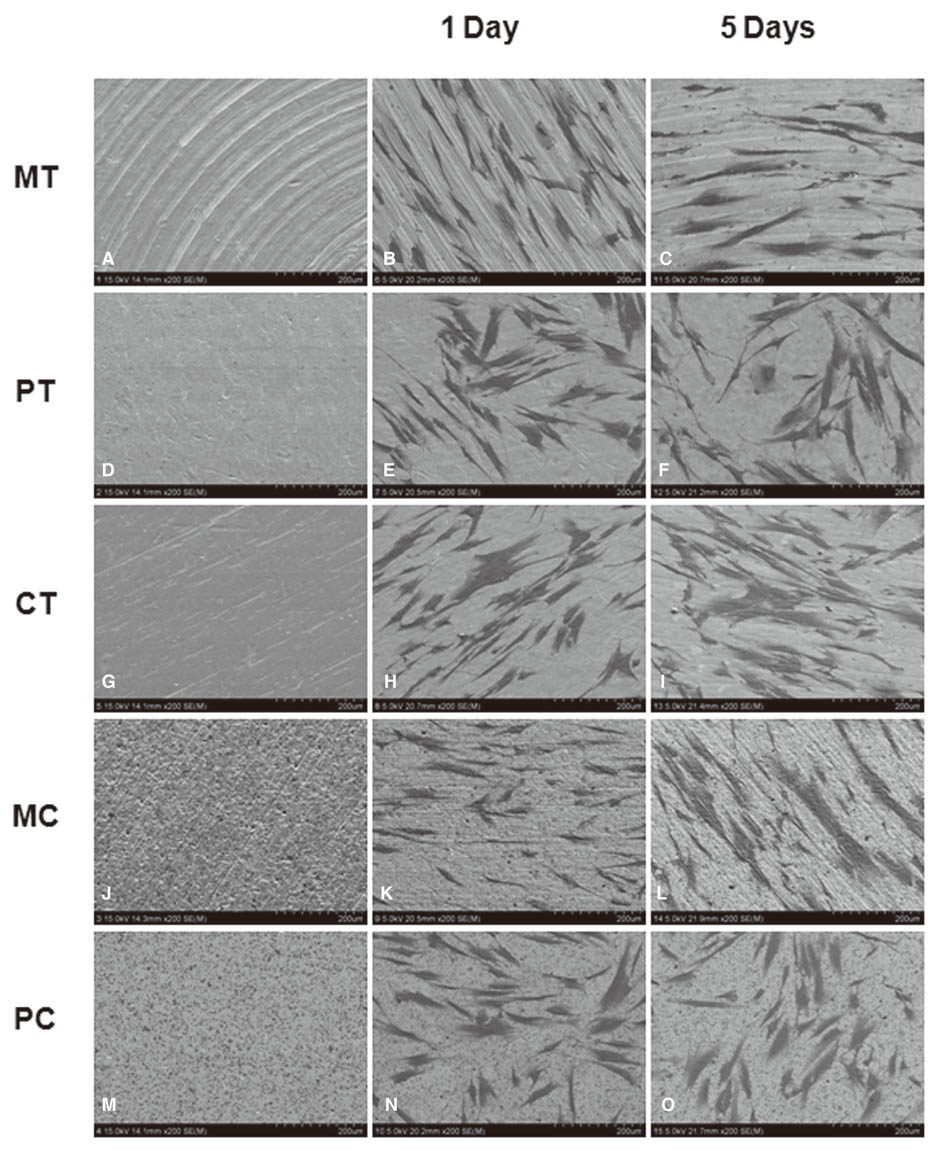
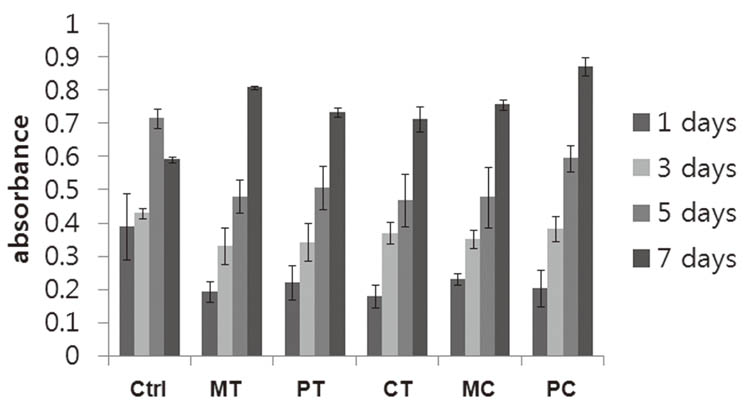
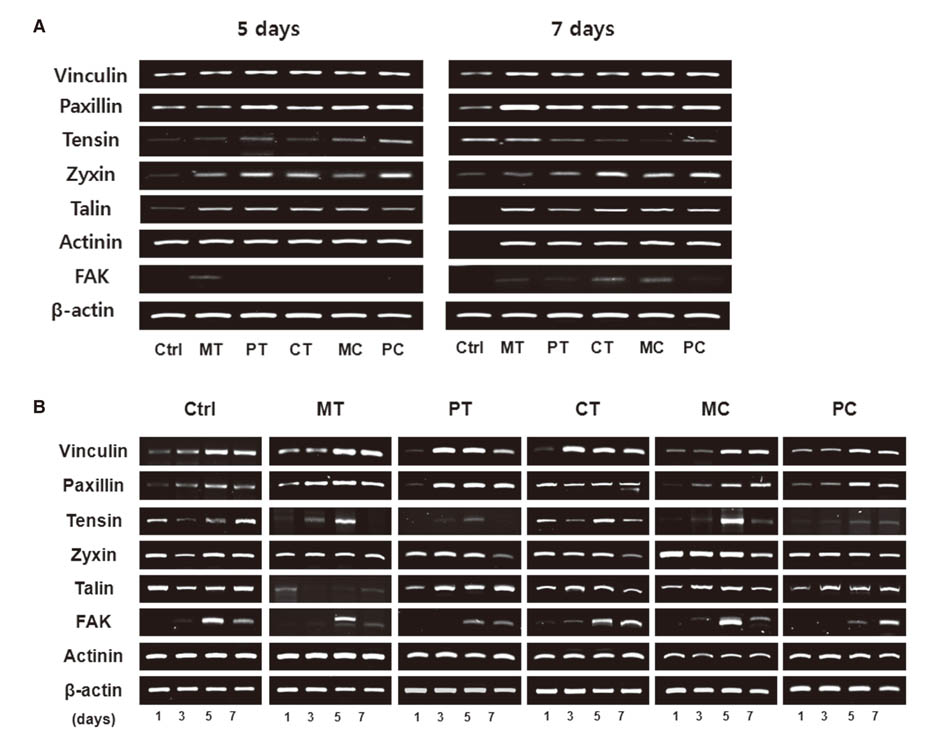
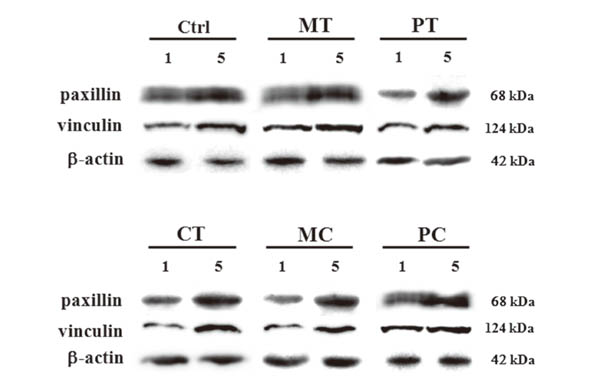
Morphologies of cultured HGFs on titanium and ceramic discs with different surface were observed by scanning electron microscopy. Biocompatibility and focal adhesion were evaluated by ultrasonic wave application and cell viability assay. FALPs expression levels were assessed by RT-PCR and western blot.
RESULTS
There seemed to be little difference in biocompatibility and adhesion strength of HGFs depending on the surface conditions and materials. In all experimental groups, the number of cells remaining on the disc surface after ultrasonic wave application increased more than 2 times at 3 days after seeding compared to 1-day cultured cells and this continued until 7 days of culture. FALPs expression levels, especially of vinculin and paxillin, also increased in 5-day cultured cells compared to 1-day cultured fibroblasts on the disc surface.
CONCLUSION
These results might suggest that the strength of adhesion of fibroblasts to transmucosal abutment surfaces increases with time and it seemed to be related to expressions of FALPs.
MeSH Terms
Figure
Reference
-
1. Palma-Carrió C, Balaguer-Martínez J, Peñarrocha-Oltra D, Peñarrocha-Diago M. Irritative and sensory disturbances in oral implantology. Literature review. Med Oral Patol Oral Cir Bucal. 2011; 16:e1043–e1046.2. Berglundh T, Lindhe J, Ericsson I, Marinello CP, Liljenberg B, Thomsen P. The soft tissue barrier at implants and teeth. Clin Oral Implants Res. 1991; 2:81–90.3. Listgarten MA, Buser D, Steinemann SG, Donath K, Lang NP, Weber HP. Light and transmission electron microscopy of the intact interfaces between non-submerged titanium-coated epoxy resin implants and bone or gingiva. J Dent Res. 1992; 71:364–371.4. Berglundh T, Lindhe J. Dimension of the peri-implant mucosa: Biological width revisited. J Clin Periodontol. 1996; 23:971–973.5. Ambrose EJ. The movements of fibrocytes. Exp Cell Res. 1961; 8:54–73.6. Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988; 4:487–525.7. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002; 110:673–687.8. Owen GR, Meredith DO, ap Gwynn I, Richards RG. Focal adhesion quantification - a new assay of material biocompatibility? Review. Eur Cell Mater. 2005; 9:85–96.9. An N, Rausch-fan X, Wieland M, Matejka M, Andrukhov O, Schedle A. Initial attachment, subsequent cell proliferation/viability and gene expression of epithelial cells related to attachment and wound healing in response to different titanium surfaces. Dent Mater. 2012; 28:1207–1214.10. Anselme K, Bigerelle M, Noel B, Dufresne E, Judas D, Iost A, Hardouin P. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J Biomed Mater Res. 2000; 49:155–166.11. Boyan BD, Lohmann CH, Dean DD, Sylvia VL, Cochran DL, Schwartz Z. Mechanisms involved in osteoblast response to implant surface morphology. Annual Rev Mater Res. 2001; 31:357–371.12. Garrod DR. Cell to cell and cell to matrix adhesion. BMJ. 1993; 306:703–705.13. Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999; 285:1028–1032.14. Räisänen L, Könönen M, Juhanoja J, Varpavaara P, Hautaniemi J, Kivilahti J, Hormia M. Expression of cell adhesion complexes in epithelial cells seeded on biomaterial surfaces. J Biomed Mater Res. 2000; 49:79–87.15. Biggs MJ, Richards RG, Gadegaard N, Wilkinson CD, Dalby MJ. Regulation of implant surface cell adhesion: characterization and quantification of S-phase primary osteoblast adhesions on biomimetic nanoscale substrates. J Orthop Res. 2007; 25:273–282.16. Arvidson K, Fartash B, Hilliges M, Köndell PA. Histological characteristics of peri-implant mucosa around Brånemark and single-crystal sapphire implants. Clin Oral Implants Res. 1996; 7:1–10.17. Abrahamsson I, Berglundh T, Glantz PO, Lindhe J. The mucosal attachment at different abutments. An experimental study in dogs. J Clin Periodontol. 1998; 25:721–727.18. Craig RG, Hanks CT. Cytotoxicity of experimental casting alloys evaluated by cell culture tests. J Dent Res. 1990; 69:1539–1542.19. Li D, Ferguson SJ, Beutler T, Cochran DL, Sittig C, Hirt HP, Buser D. Biomechanical comparison of the sandblasted and acid-etched and the machined and acid-etched titanium surface for dental implants. J Biomed Mater Res. 2002; 60:325–332.20. Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J, Lankford J Jr, Dean DD, Cochran DL, Boyan BD. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J Biomed Mater Res. 1995; 29:389–401.21. Kieswetter K, Schwartz Z, Hummert TW, Cochran DL, Simpson J, Dean DD, Boyan BD. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like MG-63 cells. J Biomed Mater Res. 1996; 32:55–63.22. Batzer R, Liu Y, Cochran DL, Szmuckler-Moncler S, Dean DD, Boyan BD, Schwartz Z. Prostaglandins mediate the effects of titanium surface roughness on MG63 osteoblast-like cells and alter cell responsiveness to 1 alpha,25-(OH)2D3. J Biomed Mater Res. 1998; 41:489–496.23. Lossdörfer S, Schwartz Z, Wang L, Lohmann CH, Turner JD, Wieland M, Cochran DL, Boyan BD. Microrough implant surface topographies increase osteogenesis by reducing osteoclast formation and activity. J Biomed Mater Res A. 2004; 70:361–369.24. Könönen M, Hormia M, Kivilahti J, Hautaniemi J, Thesleff I. Effect of surface processing on the attachment, orientation, and proliferation of human gingival fibroblasts on titanium. J Biomed Mater Res. 1992; 26:1325–1341.25. Grössner-Schreiber B, Herzog M, Hedderich J, Dück A, Hannig M, Griepentrog M. Focal adhesion contact formation by fibroblasts cultured on surface-modified dental implants: an in vitro study. Clin Oral Implants Res. 2006; 17:736–745.26. Park J, Bauer S, von der Mark K, Schmuki P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007; 7:1686–1691.27. Park J, Bauer S, Schlegel KA, Neukam FW, von der Mark K, Schmuki P. TiO2 nanotube surfaces: 15 nm-an optimal length scale of surface topography for cell adhesion and differentiation. Small. 2009; 5:666–671.28. Meredith DO, Eschbach L, Wood MA, Riehle MO, Curtis AS, Richards RG. Human fibroblast reactions to standard and electropolished titanium and Ti-6Al-7Nb, and electropolished stainless steel. J Biomed Mater Res A. 2005; 75:541–555.29. Derhami K, Wolfaardt JF, Wennerberg A, Scott PG. Quantifying the adherence of fibroblasts to titanium and its enhancement by substrate-attached material. J Biomed Mater Res. 2000; 52:315–322.30. Groessner-Schreiber B, Neubert A, Müller WD, Hopp M, Griepentrog M, Lange KP. Fibroblast growth on surface-modified dental implants: an in vitro study. J Biomed Mater Res A. 2003; 64:591–599.31. Reyes CD, García AJ. A centrifugation cell adhesion assay for high-throughput screening of biomaterial surfaces. J Biomed Mater Res A. 2003; 67:328–333.32. Hunter A, Archer CW, Walker PS, Blunn GW. Attachment and proliferation of osteoblasts and fibroblasts on biomaterials for orthopaedic use. Biomaterials. 1995; 16:287–295.33. Richards RG, Stiffanic M, Owen GR, Riehle M, Ap Gwynn I, Curtis AS. Immunogold labelling of fibroblast focal adhesion sites visualised in fixed material using scanning electron microscopy, and living, using internal reflection microscopy. Cell Biol Int. 2001; 25:1237–1249.34. Grover A, Rosentraus MJ, Sterman B, Snook ME, Adamson ED. An adhesion-defective variant of F9 embryonal carcinoma cells fails to differentiate into visceral endoderm. Dev Biol. 1987; 120:1–11.35. Rodríguez Fernández JL, Geiger B, Salomon D, Ben-Ze'ev A. Overexpression of vinculin suppresses cell motility in BALB/c 3T3 cells. Cell Motil Cytoskeleton. 1992; 22:127–134.36. Rodríguez Fernández JL, Geiger B, Salomon D, Ben-Ze'ev A. Suppression of vinculin expression by antisense transfection confers changes in cell morphology, motility, and anchorage-dependent growth of 3T3 cells. J Cell Biol. 1993; 122:1285–1294.37. Alenghat FJ, Fabry B, Tsai KY, Goldmann WH, Ingber DE. Analysis of cell mechanics in single vinculin-deficient cells using a magnetic tweezer. Biochem Biophys Res Commun. 2000; 277:93–99.38. Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol. 2000; 2:e231–e236.39. Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004; 84:1315–1339.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of beta- & alpha-Catenin and Focal Adhesion Kinase in Invasive Cervical Cancers
- Effect of laser-dimpled titanium surfaces on attachment of epithelial-like cells and fibroblasts
- Preferential Cytotoxic Effect of Genistein on G361 Melanoma Cells Via Inhibition of the Expression of Focal Adhesion Kinase
- Allicin Reduces Adhesion Molecules and NO Production Induced by gamma irradiation in Human Endothelial Cells
- Syntenin Is Expressed in Human Follicular Dendritic Cells and Involved in the Activation of Focal Adhesion Kinase