J Korean Ophthalmol Soc.
2009 Mar;50(3):424-428. 10.3341/jkos.2009.50.3.424.
The Effect of Dorzolamide-Timolol and Latanoprost on Redox Cycle in Aqueous Humor of Rabbit
- Affiliations
-
- 1Department of Ophthalmology, Chonnam National University Medical School & Hospital, Gwangju, Korea. exo70@naver.com
- KMID: 2111254
- DOI: http://doi.org/10.3341/jkos.2009.50.3.424
Abstract
-
PURPOSE: To evaluate the effect of dorzolamide-timolol and latanoprost on redox cycle of superoxide dismutase (SOD) and ascorbic acid in the aqueous humor of the rabbit.
METHODS
Group 1 (20 eyes of 10 rabbits) was instilled with dorzolamide-timolol in the right eye and Group 2 (20 eyes of 10 rabbits) was instilled with latanoprost in the same manner. Four weeks after instillation, SOD activity and ascorbic acid concentration were analyzed.
RESULTS
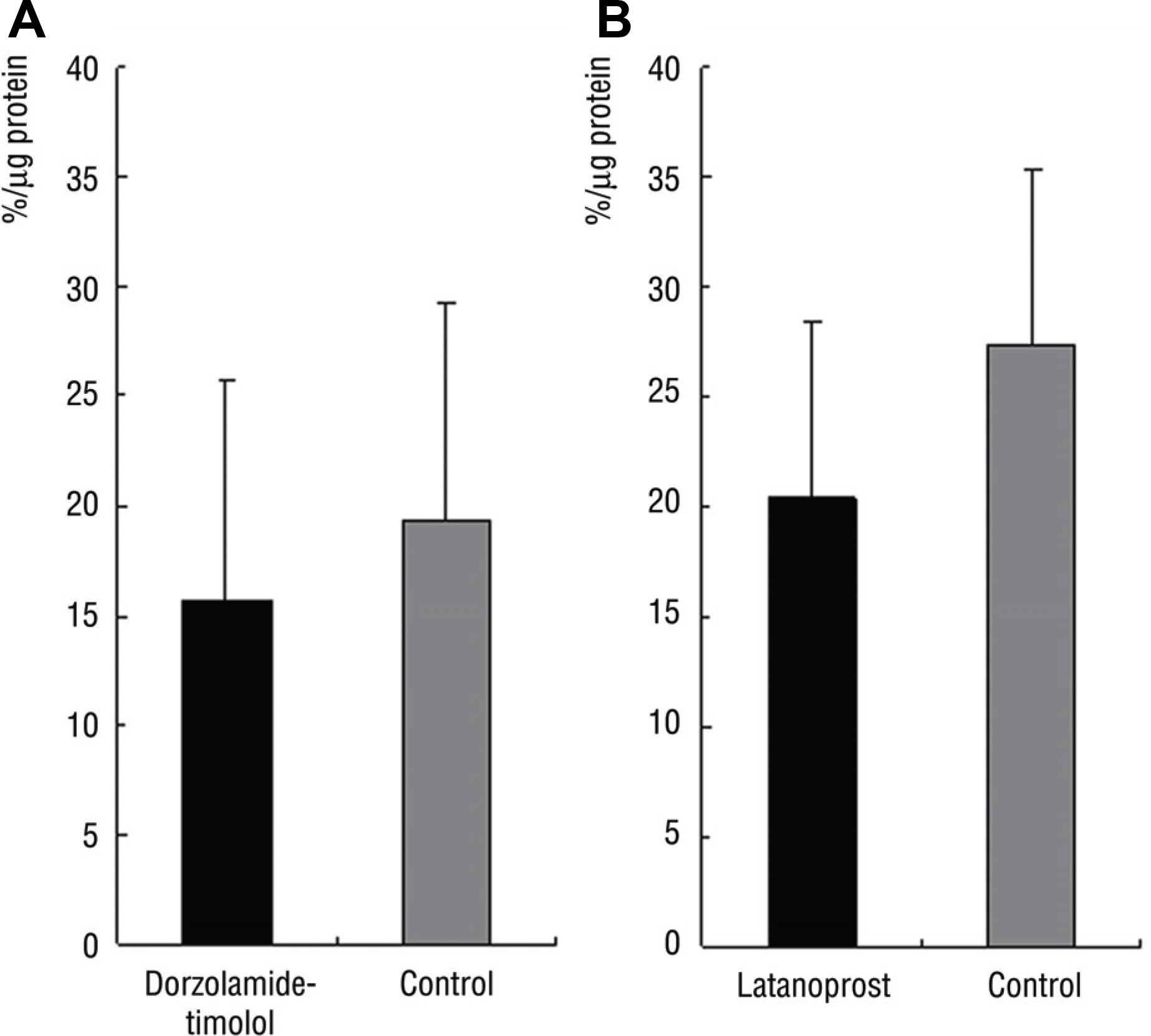
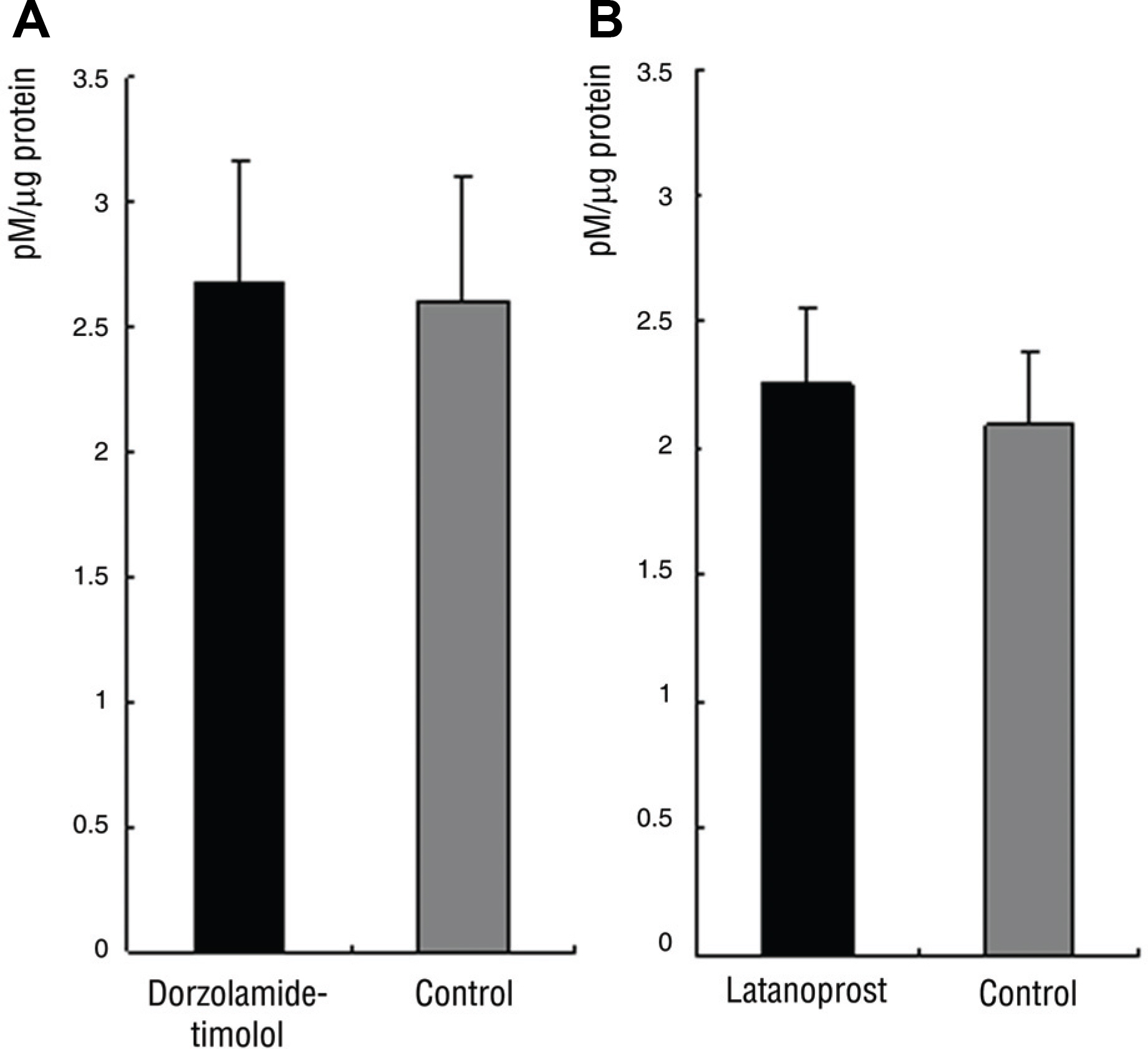
In Group 1, SOD activity and ascorbic acid concentration of the right eye and left eye were 15.7% and 19.3% (p=0.27), 2.7 pM and 2.6 pM (p=0.47), respectively. In Group 2, SOD activity and ascorbic acid concentration of the right and left eye were 22.3% and 27.6% (p=0.14), 2.3 pM and 2.1 pM (p=0.89), respectively.
CONCLUSIONS
There was no significant difference of SOD activity and ascorbic acid concentration between both eyes in Group 1 and 2.
MeSH Terms
Figure
Reference
-
References
1. Gupta N, Weinreb RN. New definition of glaucoma. Curr Opin Ophthalmol. 1997; 8:38–41.2. Quigley HA, Nickells RW, Kerrigan LA, et al. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995; 36:774–86.3. Sacca SC, Pascotto A, Camicione P, et al. Oxidative DNA damage in human trabecular meshwork. Arch Ophthalmol. 2005; 123:458–63.4. Zhou L, Li Y, Yue BY. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from an ocular tissue: the trabecular meshwork. J Cell Physiol. 1999; 180:182–9.
Article5. Rose Rc, Richer SP, Bode AM. Ocular oxidants and antioxidant protection. Proc Soc Exp Biol Med. 1998; 217:397–407.
Article6. Babizhayev MA, Bunin AY. Lipid peroxidation in open-angle glaucoma. Acta Ophthalmol. 1989; 67:371–7.
Article7. Luthra A, Gupta N, Kaufman PL, et al. Oxidative injury by perox-ynitrite in neural and vascular tissue of the lateral geniculate nucleus in experimental glaucoma. Exp Eye Res. 2005; 80:43–9.
Article8. Izzotti A, Sacca SC, Cartiglia C, De Flora S. Oxidative deoxyrib-oneucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003; 114:638–46.9. Levin LA, Clark JA, Johns LK. Effect of lipid peroxidation inhibition on retinal ganglion cell death. Invest Ophthalmol Vis Sci. 1996; 37:2744–9.10. Neufeld AH. Nitric oxide: a potential mediator of retinal ganglion cell damage in glaucoma. Surv Ophthamol. 1999; 43:129–35.11. Erden M, Bor NM. Changes of reduced glutathione, glutathione reductase, and glutathione peroxidase after radiation of guinea pigs. Biochem Med. 1984; 31:217–27.12. Richer SP, Rose RC. Water soluble antioxidants in mammalian aqueous humor: interaction with UV and hydrogen peroxide. Vision Res. 1998; 38:2881–8.13. Valencia E, Hardy G, Marin A. Glutathione: nurtritional and pharmacologic viewpoints: PartVI. Nutrition. 2002; 18:291–2.14. Lee IS, Yu YS, Kim DM, et al. Detection of specific proteins in the aqueous humor in primary open angle glaucoma. Korean J Ophthalmol. 1990; 4:1–4.15. Izzotti A, Bagnis A, Sacca SC. The role of oxidative stress in glaucoma. Mutation Research. 2006; 612:105–14.
Article16. Beit-Yannai E, Trembovler V, Solomon AS. Decrease in reducing power of aqueous humor originating from glaucomatous rabbits. Eye. 2007; 21:658–64.
Article17. Becker B. Chemical composition of human aqueous humor. Arch Ophthalmol. 1957; 57:793–800.
Article18. Mori M, Araie M, Sakurai M, et al. Effect of pilocarpine and trpicamide on blood-aqueous barrier permeability in man. Invest Ophthalmol Vis Sci. 1992; 33:416–23.19. Richter CU, Shingleton BJ, Bellows AR, et al. The development of encapsulated filtering blebs. Ophthamology. 1988; 95:1163–8.20. Herschler J, Claflin AJ, Fiorentino G. The effect of aqueous humor on the growth of subconjunctival fibroblasts in tissue culture and its implications for glaucoma surgery. Am J Ophthalmol. 1980; 89:245–9.
Article21. De La Paz MA, Epstein DL. Effect of age on superoxide dismutase activity of human trabecular meshwork. Invest Ophthalmol Vis Sci. 1996; 37:1849–53.22. Greenlund LJ, Deckwerth TL, Johnson EM Jr. Superoxide dismutase delaysneuronal apoptosis: a role for reactive oxygen sp-ecies in programmed neuronal death. Neuron. 1995; 14:303–15.23. Lee P, Lam KW, Lai M. Aqueous humor ascorbate concentration and open-angle glaucoma. Arch Ophthalmol. 1977; 95:308–10.
Article24. Yildirim O, Ates NA, Ercan B, et al. Role of oxidative stress enzymes in open-angle glaucoma. Eye. 2005; 19:580–3.
Article25. Rao Na, Thaete LG, Delmage JM, Sevanian A. Superoxide dismutase in ocular structure. Invest Ophthalmol Vis Sci. 1985; 26:1778–81.26. Ferreira SM, Lerner SF, Brunzini R, et al. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004; 137:62–9.
Article27. Reiss GR, Wernss PG, Zollman PE, Brubaker RF. Ascorbic acid levels in the aqueous humor of nocturnal and diurnal mammals. Arch Ophthalmol. 1986; 104:735–55.
Article28. Williams RN, Paterson CA. A protective role for ascorbic acid during inflammatory episodes in the eye. Exp Eye Res. 1986; 42:211–8.
Article29. Moses RA, Hort WM Jr. Adler's physiology of the eye. 8th ed.St Louis: CV Mosby;1987. p. 212–22.30. Yue BY, Higginbotham EJ, Chang IL. Ascorbic acid modulates the production of fibronectin and laminin by cells from on eye trabecular meshwork. Exp Cell Res. 1990; 187:65–8.31. Jampel HD. Ascorbic acid is cytotoxic to dividing human Tenon's capsule fibroblast: a possible contributing factor in glaucoma filtration surgery success. Arch Ophthalmol. 1990; 108:1323–5.32. Becker B. Ascorbate transfer in guinea pig eyes. Invest Ophthalmol. 1967; 6:410–5.33. Lam KW, Lee P, Fox R. Aqueous ascorbate concentration in hereditary buphthalmic rabbits. Arch Ophthalmol. 1976; 94:1565–7.
Article34. Fox RR, Lam KW, Lewen R, Lee P. Ascorbate concentration in tissues from normal and buphthalmic rabbits. J Heredity. 1982; 73:109–11.
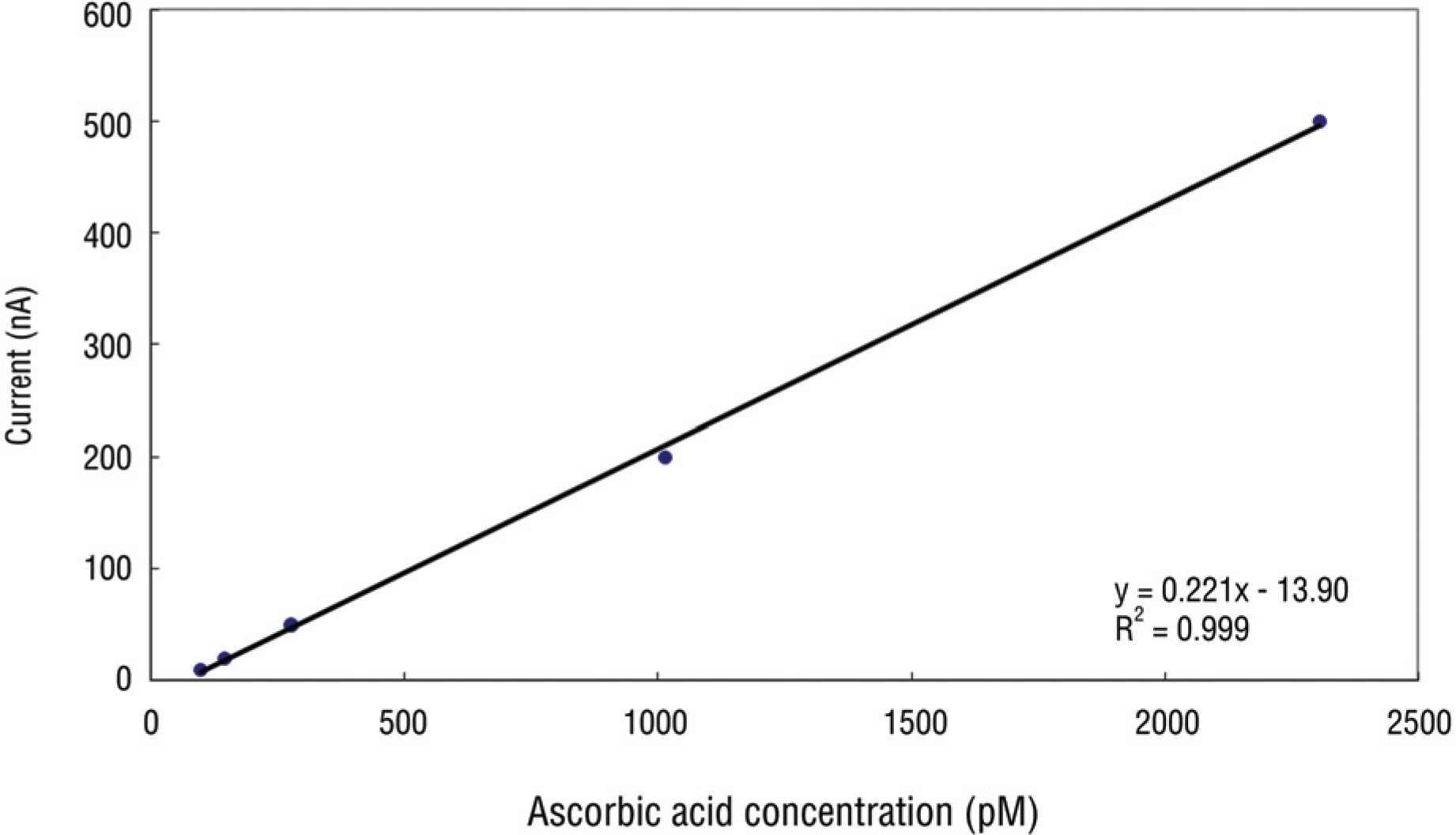
Article35. Lam KW, Lee PF. Analysis of ascorbateconcentration in the aqueous humor by high pressure liquid chromatography. Invest Ophthalmol. 1975; 14:947–50.36. Fong D, Etzel K, Lee PF, et al. Factors affecting ascorbate oxi-dation in aqueous humor. Curr Eye Res. 1987; 6:357–61.
Article37. Bar-Ilan A, Pesah NI, Maren TH. The effects of carbonic anhydrase inhibitors on aqueous humor dynamics. Invest Ophthalmol Vis Sci. 1984; 25:1198–205.38. Krohne SG. Effect of topically applied 2% pilocarpine and 0.25% demecarium bromide on blood-aqueous barrier permeability in dogs. Am J Vet Res. 1994; 55:1729–33.39. Kim DH, Kwak HW, Kim JM. Ascorbic acid determination in aqueous and vitreous humor of the rabbit. J Korean Ophthalmol Soc. 1997; 38:865–9.40. Forster S, Mead A, Sears M. An interophthalmic communicating artery as explanation for the consensual irritative response of the rabbit eye. Invest Ophthalmol Vis Sci. 1979; 18:161–5.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Studies of the Timolol Effect on Intraocular Pressure and Concentration in Aqueous Humor in the White Rabbit
- Effeet of Timolol on Aqueous Humor Protein Concentration
- Add-on Effect of Prostaglandin Analogues in Dorzolamide/Timolol Fixed Combination Treated Primary Open Angle Glaucoma Patients
- Long-term Results of Selective Laser Trabeculoplasty versus Latanoprost or Dorzolamide/Timolol Fixed Combination
- Comparison of the Intraocular Pressure Reducing E ffect of Latanoprost with Timolol