J Korean Ophthalmol Soc.
2008 Jun;49(6):1007-1012. 10.3341/jkos.2008.49.6.1007.
A Case of Retiserttrade mark Implant for Chronic Behcet's Panuveitis
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea. hgonyu@snu.ac.kr
- 2Research Center for Sensory Organs in Seoul National University Medical Research Center, Seoul, Korea.
- 3Research Center for Rheumatology in Seoul National University Medical Research Center, Seoul, Korea.
- KMID: 2110884
- DOI: http://doi.org/10.3341/jkos.2008.49.6.1007
Abstract
-
PURPOSE: Retisert(TM) (fluocinolone acetonide implant) has recently been approved for clinical use in patients with noninfectious posterior uveitis. We report a patient with intractable chronic Behcet's panuveitis who underwent Retisert(TM) implantation and showed a favorable outcome.
METHODS
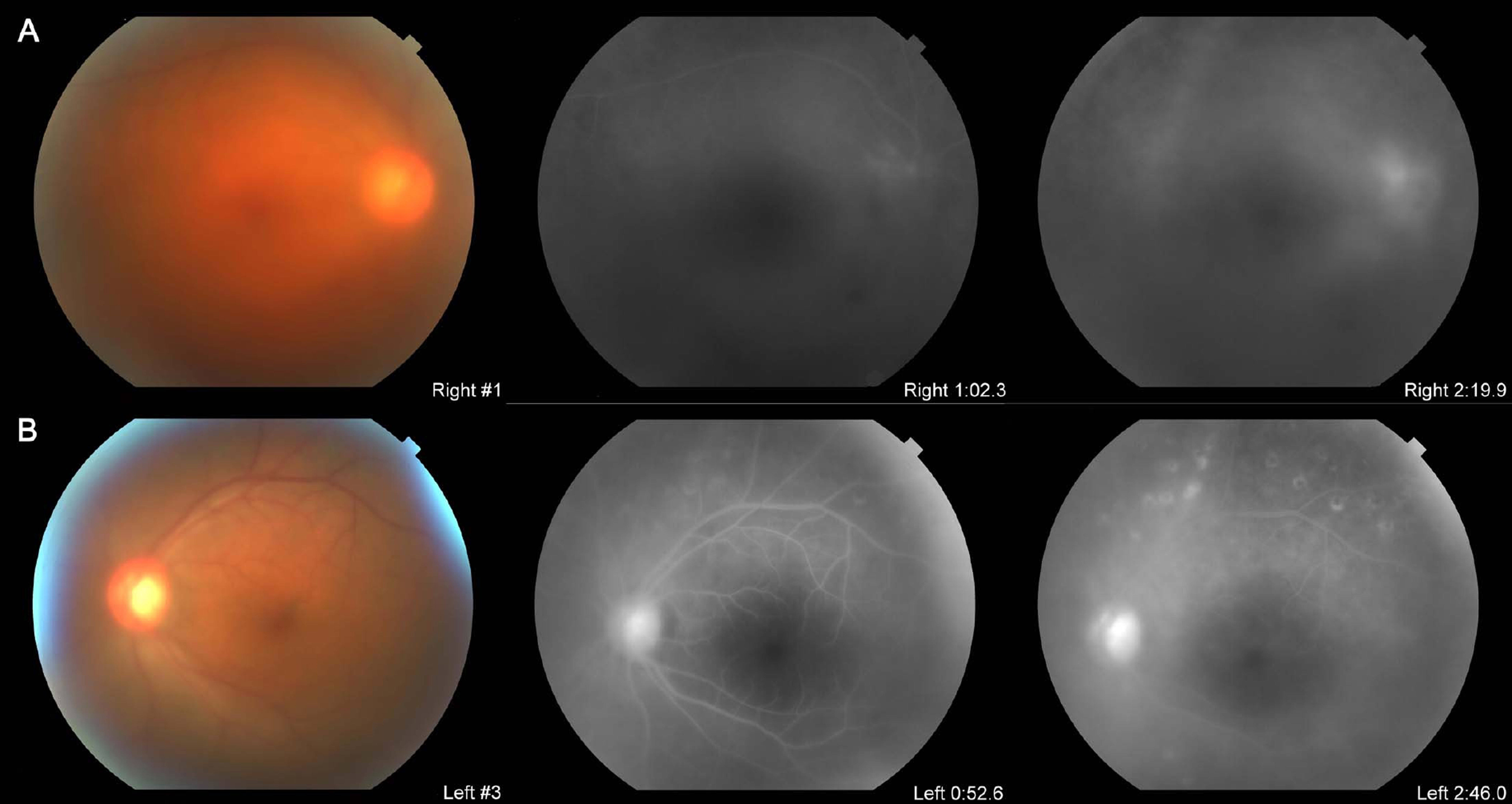
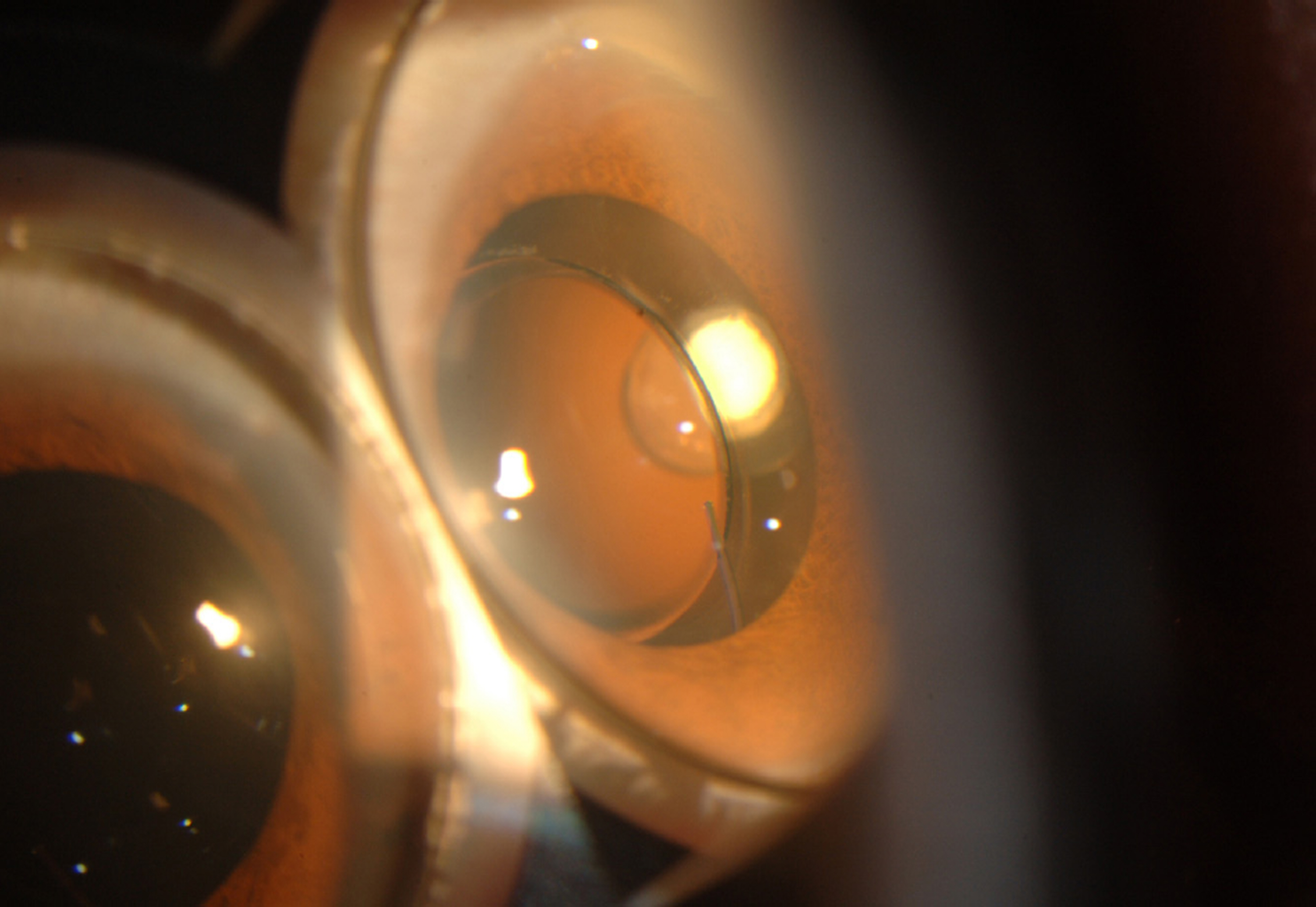
A 30-year-old male affected with intractable Behcet's uveitis of both eyes for over one year which did not respond to oral steroids and immunosuppressants; subcutaneous interferon injection caused undesirable side effects such as impotency and pyrexia. Initial visual acuities were 20/1000 in the right eye and 20/100 in the left eye, and both eyes showed severe panuveitis with posterior subcapsular cataract, especially in the right eye. The subtenon triamcinolone injection was performed in the right eye, which was only effective to anterior uveitis, and Retisert(TM) was implanted in the right eye after the cataract operation. Two months later the visual acuity increased to 20/25, and the inflammation was totally controlled. There were no ocular or systemic adverse events.
CONCLUSIONS
Retiserttrade mark is a fast, effective, and safe treatment for chronic, non.infectious posterior uveitis.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Wakefield D, Chang JH. Epidemiology of uveitis. Int Ophthalmol Clin. 2005; 45:1–13.
Article2. Nussenblatt RB, Whitcup SM, de Smet MD. . Intraocular inflammatory disease (uveitis) and the use of oral tolerance: a status report. Ann N Y Acad Sci. 1996; 778:325–37.3. Jaffe GJ, Martin D, Callanan D. . Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty- four-week results of a multicenter randomized clinical study. Ophthalmology. 2006; 113:1020–7.4. Hsu J. Drug delivery methods for posterior segment disease. Curr Opin Ophthalmol. 2007; 18:235–9.
Article5. Haupert CL, Jaffe GJ. New and emerging treatments for patients with uveitis. Int Ophthalmol Clin. 2000; 40:205–20.
Article6. Yang H, Hyun JH, Lee SC. Intravitreal triamcinolone injection for uveitic cystoid macular edema. J Korean Ophthalmol Soc. 2004; 45:1487–95.7. Choi SK, Roh YJ, Kim MH. Intravitreal injection of triamcinolone acetonide on refractory uveitis. J Korean Ophthalmol Soc. 2006; 47:396–401.8. Kim YJ, Kang SW, Ahn BH, Ham DI. The results of posterior subtenon steroid injection in uveitis patients. J Korean Ophthalmol Soc. 2003; 44:66–72.9. Jager RD, Aiello LP, Patel SC, Cunningham ET Jr. Risks of intravitreous injection: a comprehensive review. Retina. 2004; 24:676–98.
Article10. Jaffe GJ, Yang CH, Guo H. . Safety and pharmacokinetics of an intraocular fluocinolone acetonide sustained delivery device. Invest Ophthalmol Vis Sci. 2000; 41:3569–75.11. Driot JY, Novack GD, Rittenhouse KD. . Ocular pharmacokinetics of fluocinolone acetonide after Retisert intravitreal implantation in rabbits over a 1-year period. J Ocul Pharmacol Ther. 2004; 20:269–75.
Article12. Jaffe GJ, McCallum RM, Branchaud B. . Long‐ term follow‐ up results of a pilot trial of a fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology. 2005; 112:1192–8.13. Galor A, Margolis R, Kaiser PK, Lowder CY. Vitreous band formation and the sustained‐ release, intravitreal fluocinolone (Retisert) implant. Arch Ophthalmol. 2007; 125:836–8.14. Jaffe GJ, Ben‐ Nun J, Guo H. . Fluocinolone acetonide sustained drug delivery device to treat severe uveitis. Ophthalmology. 2000; 107:2024–33.
Article15. Dick AD, Azim M, Forrester JV. Immunosuppressive therapy for chronic uveitis: optimising therapy with steroids and cyclosporin A. Br J Ophthalmol. 1997; 81:1107–12.
Article16. Ufret‐ Vincenty RL, Singh RP, Lowder CY, Kaiser PK. Cytomegalovirus retinitis after fluocinolone acetonide (Retisert) implant. Am J Ophthalmol. 2007; 143:334–5.17. Nguyen QD, Callanan D, Dugel P. . Treating chronic noninfectious posterior segment uveitis: the impact of cumulative damage. Proceedings of an expert panel roundtable discussion. Retina. 2006; 1–16.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Bultifocal choroiditis and Panuveitis
- Correlation of sFas Level with Uveitis Severity
- A Case of Refractory Behcet's Uveitis Improving after Insertion of Fluocinolone Acetonide Implant
- The Clinical Classification and Characteristics of Uveitis
- A Case of Behcet's Disease Associated with Pyoderma Gangrenosum