Korean Circ J.
2011 Sep;41(9):497-502. 10.4070/kcj.2011.41.9.497.
Molecular Imaging of High-Risk Atherosclerotic Plaques: Is It Clinically Translatable?
- Affiliations
-
- 1Cardiovascular Center and Cardiology Division, Seoul St. Mary's Hospital, Seoul, Korea. kiyuk@catholic.ac.kr
- KMID: 2094100
- DOI: http://doi.org/10.4070/kcj.2011.41.9.497
Abstract
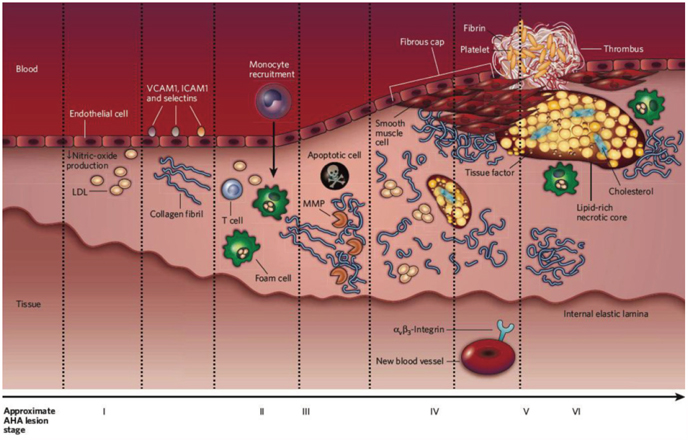
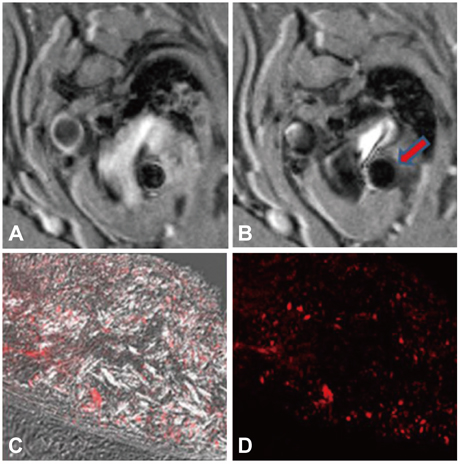
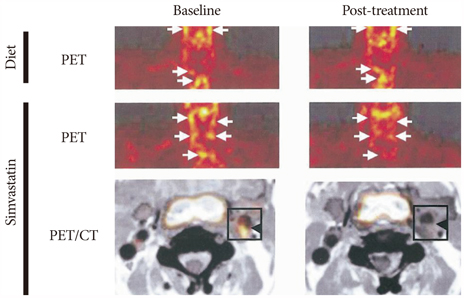
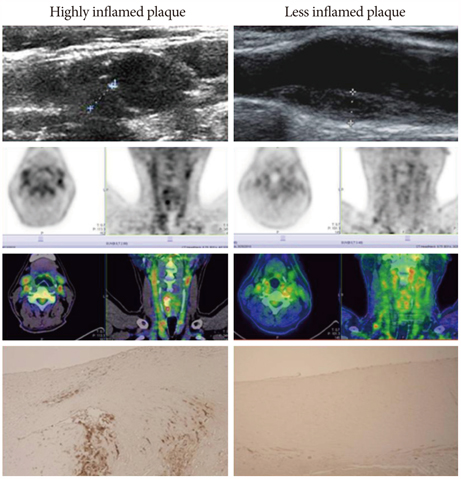
- The explosive epidemics of diabetes and obesity as well as an aging population have led to cardiovascular diseases as the leading cause of world-wide morbidity and mortality beyond cancer. The recent introduction of drug-eluting stents and medications such as statins, dual anti-platelet therapy, and angiotensin converting enzyme inhibitors has dramatically improved clinical outcomes in patients with cardiovascular diseases. However, mortality is still increasing despite state-of-the-art therapeutics, as current diagnostic and therapeutic strategies against cardiovascular disease center on "locking the barn door after the horse has been stolen". Novel diagnostic solutions that identify individuals at risk before the disease is overt are needs. Imaging approaches that visualize molecular targets rather than anatomical structures aim to illuminate vital molecular and cellular aspects of atherosclerosis biology in vivo. Recent technological advances in small animal imaging systems and dedicated targeted/activatable molecular imaging probes have positioned molecular imaging to greatly impact atherosclerosis imaging in the next decade. However, several issues must be addressed before its clinical translation.
MeSH Terms
Figure
Cited by 1 articles
-
Effect of Pioglitazone in Combination with Moderate Dose Statin on Atherosclerotic Inflammation: Randomized Controlled Clinical Trial Using Serial FDG-PET/CT
Eun Ho Choo, Eun-Ji Han, Chan Joon Kim, Sung-Hoon Kim, Joo-Hyun O, Kiyuk Chang, Ki-Bae Seung
Korean Circ J. 2018;48(7):591-601. doi: 10.4070/kcj.2017.0029.
Reference
-
1. Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009. 54:2205–2241.2. Mehta SR, Cannon CP, Fox KA, et al. Routine vs selective invasive strategies in patients with acute coronary syndrome: a collaborative meta-analysis of randomized trials. JAMA. 2005. 293:2908–2917.3. Fox KA, Poole-Wilson PA, Henderson RA, et al. Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: the British Heart Foundation RITA 3 randomized trial: randomized Intervention Trial of unstable Angina. Lancet. 2002. 360:743–751.4. Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007. 356:1503–1516.5. Wang JC, Normand SL, Mauri L, Kuntz RE. Coronary artery spatial distribution of acute myocardial infarction occlusions. Circulation. 2004. 110:278–284.6. Sinusas AJ, Bengel F, Nahrendorf M, et al. Multimodality cardiovascular molecular imaging, part I. Circ Cardiovasc Imaging. 2008. 1:244–256.7. Nahrendorf M, Sosnovik DE, French BA, et al. Multimodality cardiovascular molecular imaging, part II. Circ Cardiovasc Imaging. 2009. 2:56–70.8. Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008. 451:953–957.9. Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009. 54:2129–2138.10. Jaffer FA, Nahrendorf M, Sosnovik D, Kelly KA, Aikawa E, Weissleder R. Cellular imaging of inflammation in atherosclerosis using magnetofluorescent nanomaterials. Mol Imaging. 2006. 5:85–92.11. Trivedi RA, Mallawarachi C, U-King-Im JM, et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006. 26:1601–1606.12. Lee K, Moon HY, Park C, et al. Magnetic resonance imaging of macrophage activity in atherosclerotic plaques of apolipoprotein E-deficient mice by using polyethylene glycolated magnetic fluorescent silica-coated nanoparticles. Curr Appl Phys. 2009. 9:S15–S18.13. Rudd JH, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002. 105:2708–2711.14. Tahara N, Kai H, Ishibashi M, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006. 48:1825–1831.15. Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010. 30:1282–1292.16. Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006. 47:8 Suppl. C7–C12.17. Dollery CM, Libby P. Atherosclerosis and proteinase activation. Cardiovasc Res. 2006. 69:625–635.18. Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003. 108:1664–1672.19. Chang K, Jaffer F. Advances in fluorescence imaging of the cardiovascular system. J Nucl Cardiol. 2008. 15:417–428.20. Jaffer FA, Vinegoni C, John MC, et al. Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis. Circulation. 2008. 118:1802–1809.21. Jaffer FA, Calfon MA, Rosenthal A, et al. Two-dimensional intravascular near-infrared fluorescence molecular imaging of inflammation in atherosclerosis and stent-induced vascular injury. J Am Coll Cardiol. 2011. 57:2516–2526.22. Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010. 10:36–46.23. Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010. 107:839–850.24. Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011. 145:341–355.25. Kietselaer BL, Reutelingsperger CP, Heidendal GA, et al. Noninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid artery atherosclerosis. N Engl J Med. 2004. 350:1472–1473.26. Jeziorska M, Woolley DE. Neovascularization in early atherosclerotic lesions of human carotid arteries: its potential contribution to plaque development. Hum Pathol. 1999. 30:919–925.27. Michel JB, Virmani R, Arbustini E, Pasterkamp G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J. 2011. 32:1977–1985.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Imaging of Atherosclerosis
- Noninvasive Imaging of Atherosclerotic Plaques Using MRI and CT
- Recent Advances in the Development of PET/SPECT Probes for Atherosclerosis Imaging
- Will Molecular Optical Imaging Have Clinically Important Roles in Stroke Management, and How?
- High-Resolusion Magnetic Resonance Imaging of Carotid Atherosclerotic Plaque