J Korean Med Assoc.
2009 Feb;52(2):143-150. 10.5124/jkma.2009.52.2.143.
Molecular Imaging of Atherosclerosis
- Affiliations
-
- 1Department of Neurology, Dongguk University College of Medicine, Korea. kdongeog@duih.org, totopia@duih.org
- KMID: 1469549
- DOI: http://doi.org/10.5124/jkma.2009.52.2.143
Abstract
- Atherosclerosis is characterized by progressive accumulation of lipids and inflammatory cells within the artery wall. It is a diffuse systemic disease; however, some atherosclerotic plaques are more prone to rupture causing sudden thromboembolic vascular occlusions, while others are clinically silent. Therefore, to prevent such devastating vascular events as stroke or myocardial infarction, clinicians need to have smart tools to localize high-risk vulnerable plaques, which have been a huge challenge to date. Molecular imaging, which visualizes biologic processes at the cellular and molecular level, has a potential to assess plaque vulnerability and consequently identify high-risk patients prior to the development of the clinical events. In this review, we summarize important updates on the molecular imaging of atherosclerosis in the field of optical imaging, magnetic resonance imaging, positron emission tomography, and computerized tomography imaging.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Will Molecular Optical Imaging Have Clinically Important Roles in Stroke Management, and How?
Dong Kun Lee, Matthias Nahrendorf, Dawid Schellingerhout, Dong-Eog Kim
J Clin Neurol. 2010;6(1):10-18. doi: 10.3988/jcn.2010.6.1.10.
Reference
-
1. Kung H. Deaths: Final Data for 2005. National Vital Statistics Reports. 56.2. Libby P. Inflammation in atherosclerosis. Nature. 2002. 420:868–874.
Article3. Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008. 451:904–913.
Article4. Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999. 340:115–126.5. Lusis AJ. Atherosclerosis. Nature. 2000. 407:233–241.
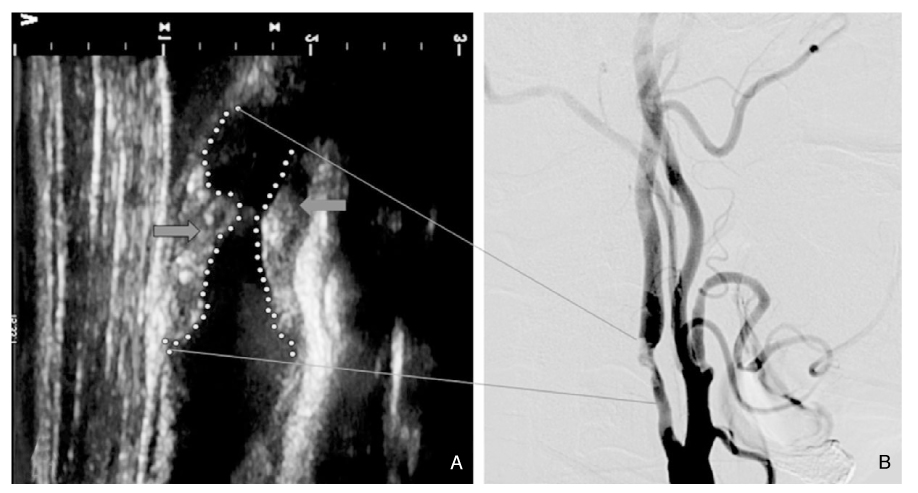
Article6. Geroulakos G, Ramaswami G, Nicolaides A, James K, Labropoulos N, Belcaro G, Holloway M. Charac-terization of symp tomatic and asymptomatic carotid plaques using highresolution real-time ultrasonography. Br J Surg. 1993. 80:1274–1277.
Article7. Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, Carroll BA, Eliasziw M, Gocke J, Hertzberg BS, Katanick S, Needleman L, Pellerito J, Polak JF, Rholl KS, Wooster DL, Zierler RE. Carotid artery stenosis: gray-scale and Doppler US diagnosis-Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003. 229:340–346.
Article8. Gronholdt ML. Ultrasound and lipoproteins as predictors of lipid-rich, rupture-prone plaques in the carotid artery. Arterioscler Thromb Vasc Biol. 1999. 19:2–13.
Article9. Gronholdt ML, Nordestgaard BG, Wiebe BM, Wilhjelm JE, Sillesen H. Echo-lucency of computerized ultrasound images of carotid atherosclerotic plaques are associated with increased levels of triglyceride-rich lipoproteins as well as increased plaque lipid content. Circulation. 1998. 97:34–40.
Article10. Jaffer FA, Libby P, Weissleder R. Molecular and cellular imaging of atherosclerosis: emerging applications. J Am Coll Cardiol. 2006. 47:1328–1338.11. Jaffer FA, Libby P, Weissleder R. Molecular imaging of cardiovascular disease. Circulation. 2007. 116:1052–1061.
Article12. Jaffer FA, Weissleder R. Molecular imaging in the clinical arena. Jama. 2005. 293:855–862.
Article13. Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001. 219:316–333.
Article14. Mayo SW, Eldrup-Jorgensen J, Lucas FL, Wennberg DE, Bredenberg CE. Carotid endarterectomy after NASCET and ACAS: a statewide study. North American Symptomatic Carotid Endarterectomy Trial. Asymptomatic Carotid Artery Stenosis Study. J Vasc Surg. 1998. 27:1017–1022. discussion 22-23.15. Moore WS, Barnett HJ, Beebe HG, Bernstein EF, Brener BJ, Brott T, Caplan LR, Day A, Goldstone J, Hobson RW 2nd, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Circulation. 1995. 91:566–579.16. Smith SC Jr, Feldman TE, Hirshfeld JW Jr, Jacobs AK, Kern MJ, King SB 3rd, Morrison DA, O'Neill WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention-summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). Circulation. 2006. 113:156–175.
Article17. Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, Thorpe KE, Meldrum HE, Spence JD. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998. 339:1415–1425.
Article18. Biller J, Feinberg WM, Castaldo JE, Whittemore AD, Harbaugh RE, Dempsey RJ, Caplan LR, Kresowik TF, Matchar DB, Toole JF, Easton JD, Adams HP Jr, Brass LM, Hobson RW 2nd, Brott TG, Sternau L. Guidelines for carotid endarterectomy: a statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Circulation. 1998. 97:501–509.19. Inzitari D, Eliasziw M, Gates P, Sharpe BL, Chan RK, Meldrum HE, Barnett HJ. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 2000. 342:1693–1700.
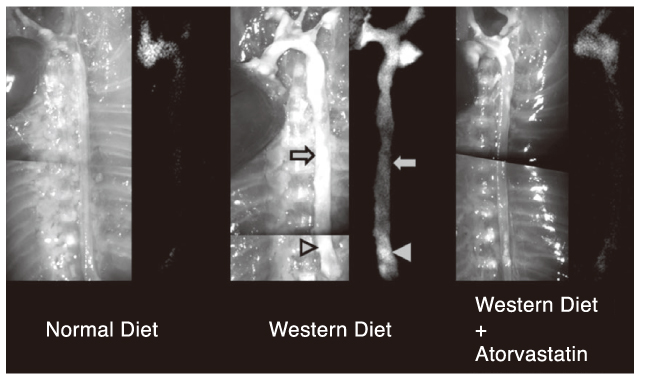
Article20. Jaffer FA, Kim DE, Quinti L, Tung CH, Aikawa E, Pande AN, Kohler RH, Shi GP, Libby P, Weissleder R. Optical visualization of cathepsin K activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007. 115:2292–2298.
Article21. Aikawa M, Libby P. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach. Cardiovasc Pathol. 2004. 13:125–138.22. Chen J, Tung CH, Mahmood U, Ntziachristos V, Gyurko R, Fishman MC, Huang PL, Weissleder R. In vivo imag-ing of proteolytic activity in atherosclerosis. Circulation. 2002. 105:2766–2771.
Article23. Deguchi JO, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, Weissleder R, Libby P. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006. 114:55–62.24. Kim DE, Kim JY, Kim EJ, Jeong SW. Molecular Optical Imaging of Cathepsin-B Proteolytic Enzyme Activity to Reflect Atherosclerosis Pathophysiology and Anti-Atherosclerotic Therapeutic Effect. J Korean Neurol Assoc. 2009. in press.25. Moon WK, Moon WK. Molecular MR Imaging. J Korean Med Assoc. 2004. 47:133–138.
Article26. Sosnovik DE, Nahrendorf M, Weissleder R. Molecular magnetic resonance imaging in cardiovascular medicine. Circulation. 2007. 115:2076–2086.
Article27. Kooi ME, Cappendijk VC, Cleutjens KB, Kessels AG, Kitslaar PJ, Borgers M, Frederik PM, Daemen MJ, van Engelshoven JM. Accumulation of ultrasmall super-paramagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003. 107:2453–2458.
Article28. Müller K, Skepper JN, Tang TY, Graves MJ, Patterson AJ, Corot C, Lancelot E, Thompson PW, Brown AP, Gillard JH. Atorvastatin and uptake of ultrasmall superparamagnetic iron oxide nanoparticles (Ferumoxtran-10) in human monocyte-macrophages: implications for magnetic resonance imaging. Biomaterials. 2008. 29:2656–2662.
Article29. Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994. 264:569–571.
Article30. Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, Allen JS, Lacy EK, Robertson JD, Lanza GM, Wickline SA. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha (v) beta3-integrin-targeted nanoparticles. Circulation. 2003. 108:2270–2274.
Article31. Chen JW, Pham W, Weissleder R, Bogdanov A Jr. Human myeloperoxidase: a potential target for molecular MR imaging in atherosclerosis. Magn Reson Med. 2004. 52:1021–1028.
Article32. Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006. 114:1504–1511.
Article33. Chu B, Kampschulte A, Ferguson MS, Kerwin WS, Yarnykh VL, O'Brien KD, Polissar NL, Hatsukami TS, Yuan C. Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke. 2004. 35:1079–1084.
Article34. Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, Hatsukami TS, Yuan C. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005. 25:234–239.
Article35. Larose E, Kinlay S, Selwyn AP, Yeghiazarians Y, Yucel EK, Kacher DF, Libby P, Ganz P. Improved characterization of atherosclerotic plaques by gadolinium contrast during intravascular magnetic resonance imaging of human arteries. Atherosclerosis. 2008. 196:919–925.
Article36. Larose E, Yeghiazarians Y, Libby P, Yucel EK, Aikawa M, Kacher DF, Aikawa E, Kinlay S, Schoen FJ, Selwyn AP, Ganz P. Characterization of human atherosclerotic plaques by intravascular magnetic resonance imaging. Circulation. 2005. 112:2324–2331.
Article37. Davies JR, Rudd JH, Fryer TD, Graves MJ, Clark JC, Kirkpatrick PJ, Kooi ME, Cappendijk VC, Cleutjens KB, Kessels AG, Kitslaar PJ, Borgers M, Frederik PM, Daemen MJ, van Engelshoven JM. Identification of culprit lesions after transient ischemic attack by combined [18F]-fluorodeoxyglucose positron emission tomography and high-resolution magnetic resonance imaging. Stroke. 2005. 36:2642–2647.
Article38. Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, Johnström P, Davenport AP, Kirkpatrick PJ, Arch BN, Pickard JD, Weissberg PL. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002. 105:2708–2711.
Article39. Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischman AJ. In vivo 18F-fluorode-oxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006. 48:1818–1824.
Article40. Kircher MF, Grimm J, Swirski FK, Libby P, Gerszten RE, Allport JR, Weissleder R. Noninvasive in vivo imaging of monocyte trafficking to atherosclerotic lesions. Circulation. 2008. 117:388–395.
Article41. Hyafil F, Cornily JC, Feig JE, Gordon R, Vucic E, Amirbekian V, Fisher EA, Fuster V, Feldman LJ, Fayad ZA. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007. 13:636–641.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Imaging of High-Risk Atherosclerotic Plaques: Is It Clinically Translatable?
- Nuclear Molecular Imaging for Vulnerable Atherosclerotic Plaques
- Molecular Optical Imaging of Cathepsin-B Proteolytic Enzyme Activity to Reflect Atherosclerosis Pathophysiology and Anti-Atherosclerotic Therapeutic Effect
- Molecular Biology of Atherosclerosis
- Molecular Optical Imaging in Neuroscience