Korean J Physiol Pharmacol.
2008 Feb;12(1):31-35. 10.4196/kjpp.2008.12.1.31.
Lysophosphatidylcholine Increases Ca2+ Current via Activation of Protein Kinase C in Rabbit Portal Vein Smooth Muscle Cells
- Affiliations
-
- 1Department of Physiology, Yonsei University College of Medicine, Seoul 120-752, Korea. dsahn@yumc.yonsei.ac.kr
- KMID: 2071643
- DOI: http://doi.org/10.4196/kjpp.2008.12.1.31
Abstract
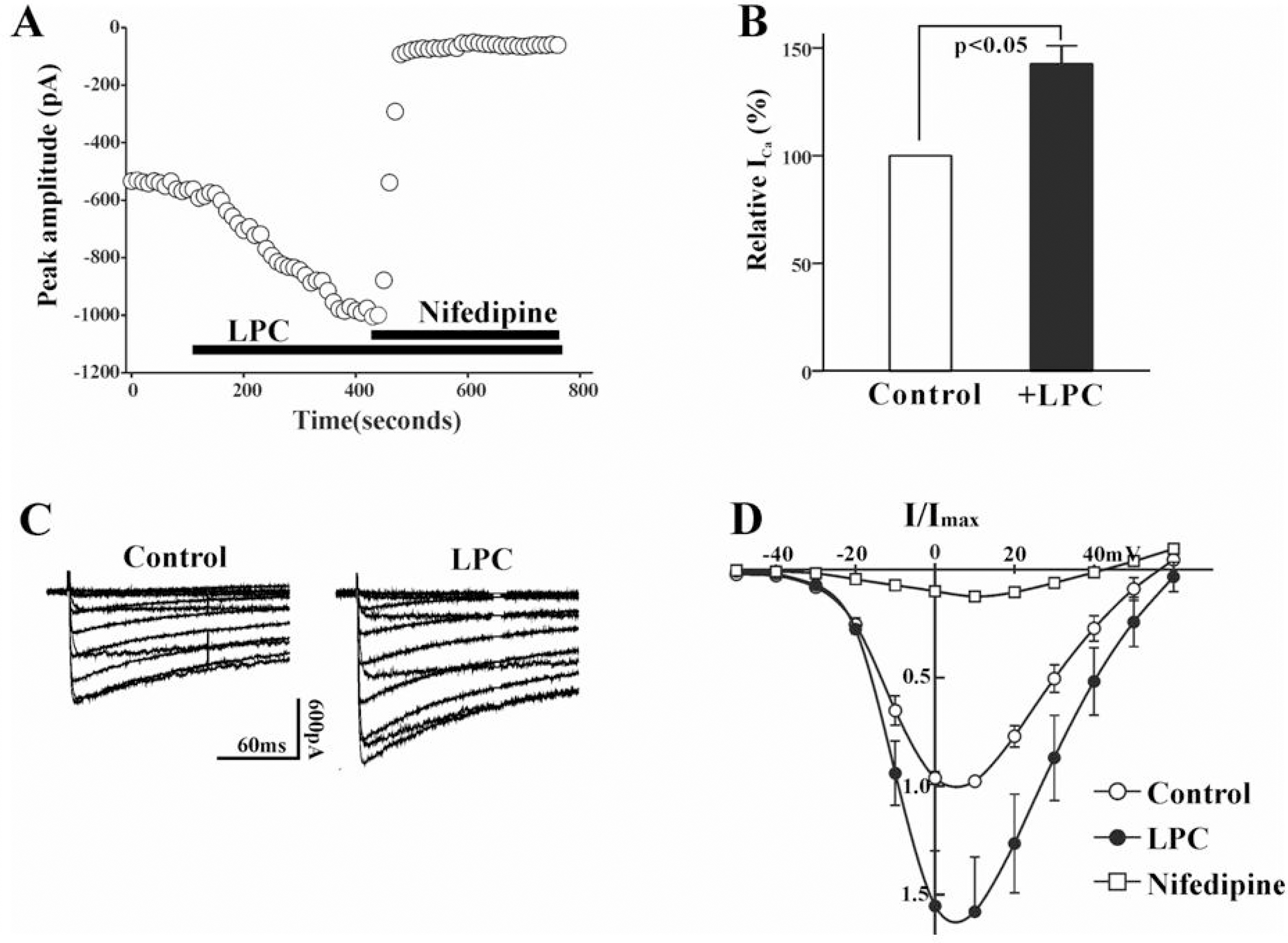
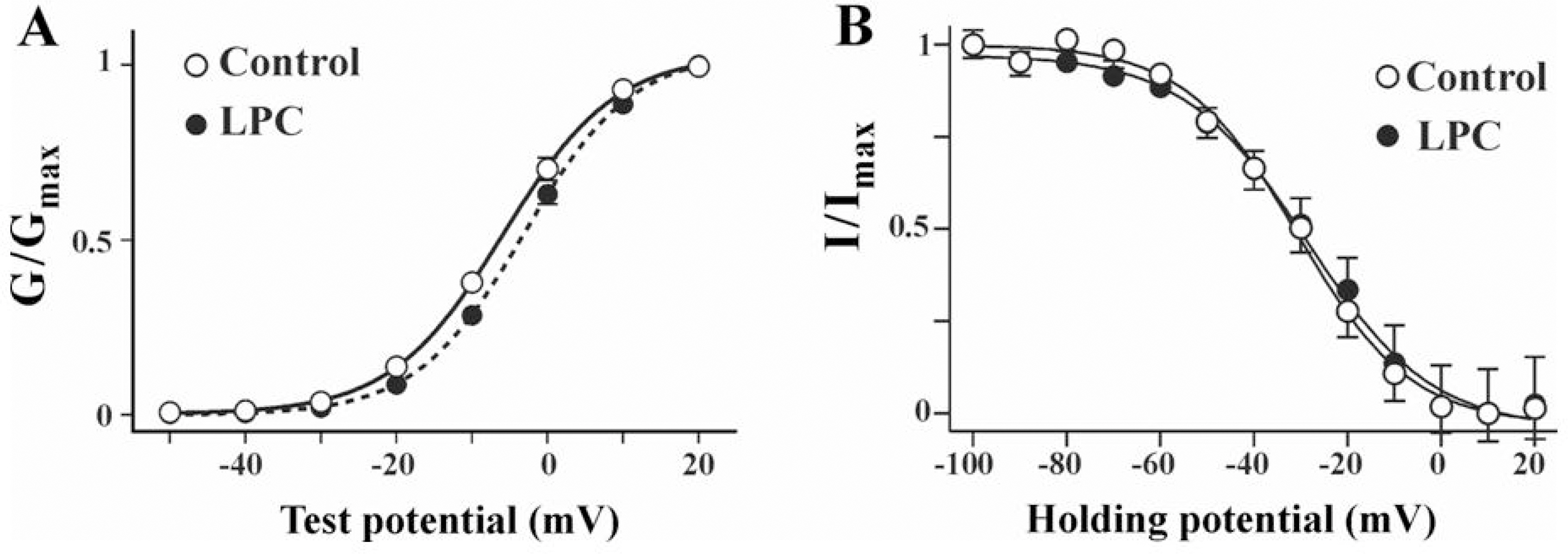
- Lysophosphatidylcholine (LPC), a metabolite of membrane phospholipids by phospholipase A(2), has been considered responsible for the development of abnormal vascular reactivity during atherosclerosis. Ca2+ influx was shown to be augmented in atherosclerotic artery which might be responsible for abnormal vascular reactivity. However, the mechanism underlying Ca2+ influx change in atherosclerotic artery remains undetermined. The purpose of the present study was to examine the effects of LPC on L-type Ca2+ current (ICa(L)) activity and to elucidate the mechanism of LPC-induced change of ICa(L) in rabbit portal vein smooth muscle cells using whole cell patch clamp. Extracellular application of LPC increased ICa(L) through whole test potentials, and this effect was readily reversed by washout. Steady state voltage dependency of activation or inactivation properties of ICa(L) was not significantly changed by LPC. Staurosporine (100 nanometer) or chelerythrine (3 micrometer, which is a potent inhibitor of PKC, significantly decreased basal ICa(L), and LPC-induced increase of ICa(L) was significantly suppressed in the presence of PKC inhibitors. On the other hand, application of PMA, an activator of PKC, increased basal ICa(L) significantly, and LPC-induced enhancement of ICa(L) was abolished by pretreatment of the cells with PMA. These findings suggest that LPC increased ICa(L) in vascular smooth muscle cells by a pathway that involves PKC, and that LPC-induced increase of ICa(L) might be, at least in part, responsible for increased Ca2+ influx in atherosclerotic artery.
MeSH Terms
-
Arteries
Atherosclerosis
Benzophenanthridines
Dependency (Psychology)
Hand
Lysophosphatidylcholines
Membranes
Muscle, Smooth
Muscle, Smooth, Vascular
Myocytes, Smooth Muscle
Phospholipases
Phospholipids
Portal Vein
Protein Kinase C
Protein Kinases
Staurosporine
Benzophenanthridines
Lysophosphatidylcholines
Phospholipases
Phospholipids
Protein Kinase C
Protein Kinases
Staurosporine
Figure
Reference
-
Auge N., Fitoussi G., Bascand JL., Pieraggi M., Junquero D., Valet P. Mildly oxidized LDL evokes a sustained Ca2+-dependent retraction of vascular smooth muscle cells. Circ Res. 79:871–880. 1996.Chen Y., Morimoto S., Kitano S., Koh E., Fukuo K., Jiang B., Chen S., Yasuda O., Hirotani A., Ogihara T. Lysophosphatidylcholine causes Ca2+ influx, enhanced DNA synthesis and cytotoxicity in cultured vascular smooth muscle cells. Atherosclerosis. 112:69–76. 1995.Clement-Chomienne O., Walsh MP., Cole WC. Antiotensin II activation of protein kinase C decreases delayed rectifier K+ current in rabbit vascular myocytes. J Physiol. 495:689–700. 1996.Cole WC., Clement-Chomienne O., Aiello EA. Regulation of 4-aminopyridine-sensitive, delayed rectifier K+ channels in vascular smooth muscle by phosphorylation. Biochem Cell Biol. 74:439–447. 1996.Cox DA., Cohen ML. Effects of oxidized low-density lipoprotein on vascular contraction and relaxation: clinical and pharmacological implications in atherosclerosis. Pharmacol Rev. 48:3–19. 1996a.Cox DA., Cohen ML. Selective enhancement of 5-hyroxytryptamine-induced contraction of porcine coronary artery by oxidized low density lipoprotein. J Pharmacol Exp Ther. 276:1095–1103. 1996b.Cox RH., Tulenko TN. Altered contractile and ion channel function in rabbit protal vein with dietary atherosclerosis. Am J Physiol. 268:H2522–H2530. 1995.Eizawa H., Yui Y., Inoue R., Kosuga K., Hattori R., Aoyama T. Lysophosphatidylcholine inhibits endothelium-dependent hyper-polarization and N-omega-nitro-L-arginine/indomethacin-resistant endothelium-dependent relaxation in the porcine coronary artery. Circulation. 92:3520–3526. 1995.Epand RM., Lester DS. The role of membrane biophysical properties in the regulation of protein kinase C activity. Trends Pharmacol Sci. 11:317–320. 1990.
ArticleGalle J., Bassenge E., Busse R. Oxidized low density lipoproteins potentiate vasoconstriction to various agonists by direct interaction with vascular smooth muscle. Circ Res. 66:1287–1293. 1990.Hoffmann J. The potential for isoenzyme-selective modulation of protein kinase C. FASEB J. 11:649–669. 1997.Jabr RI., Yamazaki J., Hume JR. Lysophosphatidylcholine triggers intracellular calcium release and activation of non-selective cation channels in renal arterial smooth muscle cells. Pflu Arch Eur J Physiol. 439:495–500. 2000.
ArticleKeef KD., Hume JR., Zhong J. Regulation of cardiac and smooth muscle Ca2+ channels by protein kinases. Am J Physiol. 281:C1743–C1756. 2001.Lepretre N., Mironneau J. Alpha 2-adrenoreceptors activate dihydropyridine-sensitive calcium channels via Gi-proteins and protein kinase C in rat portal vein myocytes. Pflu Arch. 429:253–261. 1994.Magishi K., Kimura J., Kubo Y., Abiko Y. Exogenous lysophosphatidylcholine increases non-selective cation current in guinea-pig ventricular myocytes. Pflügers Arch Eur J Physiol. 432:345–350. 1996.
ArticleMcHowat J., Yamada KA., Wu J., Yan GX., Corr BB. Recent insights pertaining to sarcolemmal phospholipid alterations underlying arrhythmogenesis in the ischemic heart. J Cardiovasc Electrophysiol. 4:288–310. 1993.
ArticleMurohara T., Kugiyama K., Ohgushi M., Sugiyama S., Ohta Y., Yasue H. LPC in oxidized LDL elicits vasocontraction and inhibits endothelium-dependent relaxation. Am J Physiol. 267:H2441–H2449. 1994.Murohara T., Scalia R., Lefer AM. Lysophosphatidylcholine promotes P-selectin expression in platelets and endothelial cells: possible involvement of protein kinase C activation and its inhibition by nitric oxide donors. Circ Res. 78:780–789. 1996.Obejero-Paz CA., Auslender M., Scarpa A. PKC activity modulates availability and long openings of L-type Ca2+ channels in A7r5 cells. Am J Physiol. 275:C535–C543. 1998.Ruegg UT., Burgess GN. Staurosporine, K-252 and NCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci. 10:218–220. 1989.Shimamura K., Kusaka M., Sperelakis N. Protein kinase C stimulates Ca2+ current in pregnant rat myometrial cells. Can J Physiol Pharmacol. 72:1304–1307. 1994.Stoll LL., Spector AA. Lysophosphatidylcholine causes cGMP-dependent verapamil-sensitive Ca2+ influx in vascular smooth muscle cells. Am J Physiol. 264:C885–C893. 1993.Sugiyama S., Kugiyama K., Ohgushi M., Fujimoto K., Yasue H. Lysophosphatidylcholine in oxidized low-density lipoprotein increases endothelial susceptibility to polymorphonuclear leukocyte-induced endothelial dysfunction in porcine coronary arteries: Role of protein kinase C. Circ Res. 74:565–575. 1994.
ArticleWatson CL., Gold MR. Lysophosphatidylcholine modulates cardiac INa via multiple protein kinase pathways. Circ Res. 81:387–395. 1997.Yeon DS., Kwon SC., Nam TS., Ahn DS. Lysophosphatidylcholine decreases delayed rectifier K current in rabbit coronary smooth muscle cells. J Vet Med Sci. 63:395–399. 2001.
ArticleZheng T., Li W., Altura BT., Altura BM. Use of protein kinase C inhibitors in rapid [Mg2+]i mobilization in primary cultured rat aortic smooth muscle cells: are certain protein kinase C isoforms natural homeostatic regulators of cytosolic free Mg2+? Eur J Pharmacol. 413:R1–R3. 2001.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of PKC-dependent Change of K+ Current Activity on Histamine-induced Contraction of Rabbit Coronary Artery
- The Effect of Activation of Protein Kinase C on the Calcium-dependent K; Current in Rat Basilar Smooth Muscle Cells
- Activation of Ca(2+)-activated K+ channels by beta agonist in rabbit coronary smooth muscle cells
- Regulation of Protein Kinase in KCl-induced Contraction of Cat Gastric Smooth Muscle
- Attenuation of Hemoglobin-Induced Constriction in the Rabbit Basilar Artery by Protein Kinase C Inhibitor and Ca2+/Calmodulin-Dependent Protein Kinase Inhibitor