J Korean Med Sci.
2014 Sep;29(Suppl 2):S123-S130. 10.3346/jkms.2014.29.S2.S123.
Urinary Sodium Excretion Has Positive Correlation with Activation of Urinary Renin Angiotensin System and Reactive Oxygen Species in Hypertensive Chronic Kidney Disease
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. mednep@snubh.org
- 2Department of Immunology, Seoul National University Postgraduate School, Seoul, Korea.
- 3Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 4Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Dongguk University Ilsan Hospital, Goyang, Korea.
- 6Department of Internal Medicine, Kyung Hee University Medical Center, Seoul, Korea.
- 7Department of Internal Medicine, Seoul St. Mary's Hospital, Seoul, Korea.
- 8Department of Internal Medicine, Seoul National University Boramae Medical Center, Seoul, Korea.
- 9Research Institute of Salt and Health, Seoul, Korea.
- 10Seoul K-Clinic, Seoul, Korea.
- KMID: 2069803
- DOI: http://doi.org/10.3346/jkms.2014.29.S2.S123
Abstract
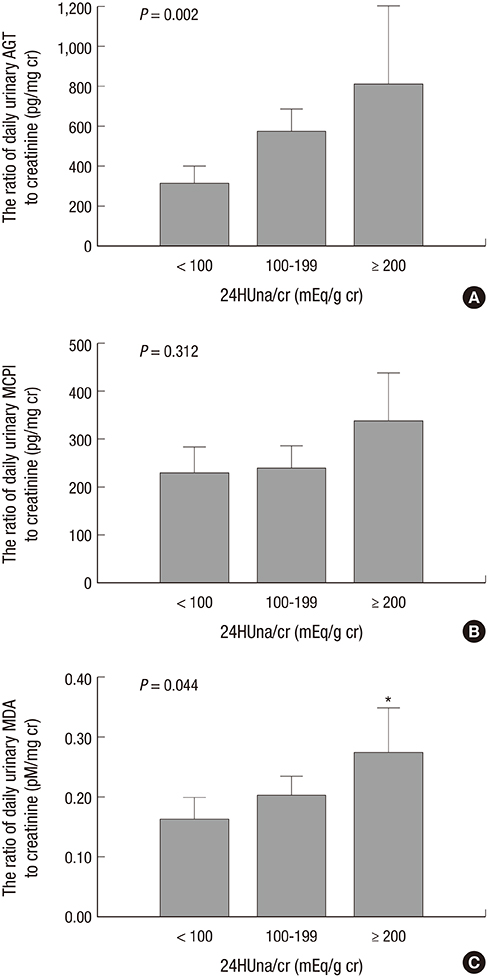
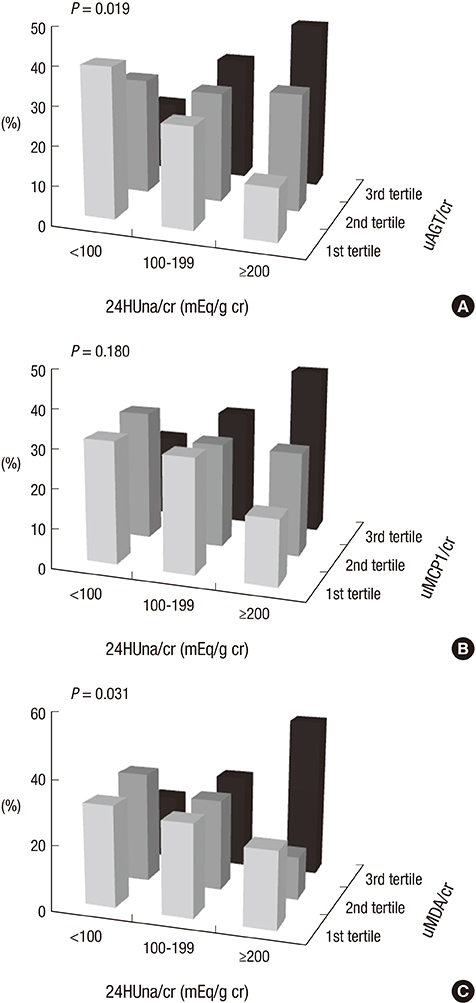
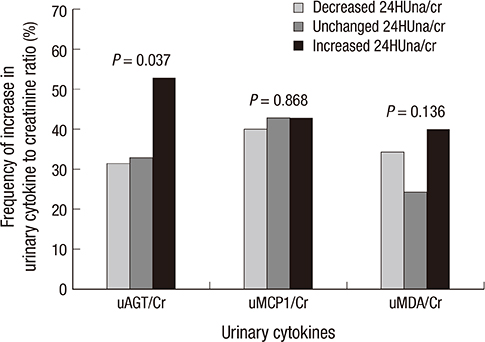
- It is not well described the pathophysiology of renal injuries caused by a high salt intake in humans. The authors analyzed the relationship between the 24-hr urine sodium-to-creatinine ratio (24HUna/cr) and renal injury parameters such as urine angiotensinogen (uAGT/cr), monocyte chemoattractant peptide-1 (uMCP1/cr), and malondialdehyde-to-creatinine ratio (uMDA/cr) by using the data derived from 226 hypertensive chronic kidney disease patients. At baseline, the 24HUna/cr group or levels had a positive correlation with uAGT/cr and uMDA/cr adjusted for related factors (P<0.001 for each analysis). When we estimated uAGT/cr in the 24HUna/cr groups by ANCOVA, the uAGT/cr in patients with > or =200 mEq/g cr was higher than in patients with <100 mEq/g cr (708 [95% CI, 448-967] vs. 334 [95% CI, 184-483] pg/mg cr, P=0.014). Similarly, uMDA/cr was estimated as 0.17 (95% CI, 0.14-0.21) pM/mg cr in patients with <100 mEq/g cr and 0.27 (95% CI, 0.20-0.33) pM/mg cr in patients with > or =200 mEq/g cr (P=0.016). During the 16-week follow-up period, an increase in urinary sodium excretion predicted an increase in urinary angiotensinogen excretion. In conclusion, high salt intake increases renal renin-angiotensin-system (RAS) activation, primarily, and directly or indirectly affects the production of reactive oxygen species through renal RAS activation.
MeSH Terms
-
Adult
Aged
Angiotensinogen/urine
Chemokine CCL2/urine
Creatine/urine
Demography
Female
Follow-Up Studies
Humans
Hypertension/complications
Male
Malondialdehyde/urine
Middle Aged
Reactive Oxygen Species/*metabolism
Renal Insufficiency, Chronic/complications/*pathology
Renin-Angiotensin System/*physiology
Sodium, Dietary/*urine
Urine Specimen Collection
Angiotensinogen
Chemokine CCL2
Creatine
Reactive Oxygen Species
Sodium, Dietary
Malondialdehyde
Figure
Reference
-
1. Kim S, Lim CS, Han DC, Kim GS, Chin HJ, Kim SJ, Cho WY, Kim YH, Kim YS. The prevalence of chronic kidney disease (CKD) and the associated factors to CKD in urban Korea: a population-based cross-sectional epidemiologic study. J Korean Med Sci. 2009; 24:S11–S21.2. Krikken JA, Laverman GD, Navis G. Benefits of dietary sodium restriction in the management of chronic kidney disease. Curr Opin Nephrol Hypertens. 2009; 18:531–538.3. Vegter S, Perna A, Postma MJ, Navis G, Remuzzi G, Ruggenenti P. Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol. 2012; 23:165–173.4. Lambers Heerspink HJ, Holtkamp FA, Parving HH, Navis GJ, Lewis JB, Ritz E, de Graeff PA, de Zeeuw D. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012; 82:330–337.5. Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ. 2007; 334:885–888.6. McMahon EJ, Bauer JD, Hawley CM, Isbel NM, Stowasser M, Johnson DW, Campbell KL. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol. 2013; 24:2096–2103.7. Drüeke TB, Parfrey PS. Summary of the KDIGO guideline on anemia and comment: reading between the (guide)line(s). Kidney Int. 2012; 82:952–960.8. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997; 349:1857–1863.9. Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, Lesti M, Perticucci E, Chakarski IN, Leonardis D, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005; 365:939–946.10. De Nicola L, Minutolo R, Chiodini P, Zoccali C, Castellino P, Donadio C, Strippoli M, Casino F, Giannattasio M, Petrarulo F, et al. Global approach to cardiovascular risk in chronic kidney disease: reality and opportunities for intervention. Kidney Int. 2006; 69:538–545.11. Sanders PW. Salt intake, endothelial cell signaling, and progression of kidney disease. Hypertension. 2004; 43:142–146.12. Ying WZ, Aaron K, Sanders PW. Mechanism of dietary salt-mediated increase in intravascular production of TGF-beta1. Am J Physiol Renal Physiol. 2008; 295:F406–F414.13. Ying WZ, Sanders PW. The interrelationship between TGF-beta1 and nitric oxide is altered in salt-sensitive hypertension. Am J Physiol Renal Physiol. 2003; 285:F902–F908.14. Franco M, Martínez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodríguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2007; 293:R251–R256.15. Tian N, Gu JW, Jordan S, Rose RA, Hughson MD, Manning RD Jr. Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007; 292:H1018–H1025.16. Chandramohan G, Bai Y, Norris K, Rodriguez-Iturbe B, Vaziri ND. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol. 2008; 28:158–167.17. Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning RD Jr. Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007; 293:H3388–H3395.18. Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol. 2003; 14:2775–2782.19. Nagase M, Matsui H, Shibata S, Gotoda T, Fujita T. Salt-induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: role of oxidative stress. Hypertension. 2007; 50:877–883.20. Sharma K, Ziyadeh FN, Alzahabi B, McGowan TA, Kapoor S, Kurnik BR, Kurnik PB, Weisberg LS. Increased renal production of transforming growth factor-beta1 in patients with type II diabetes. Diabetes. 1997; 46:854–859.21. Ellis D, Forrest KY, Erbey J, Orchard TJ. Urinary measurement of transforming growth factor-beta and type IV collagen as new markers of renal injury: application in diabetic nephropathy. Clin Chem. 1998; 44:950–956.22. Yu W, Luying S, Haiyan W, Xiaomei L. Importance and benefits of dietary sodium restriction in the management of chronic kidney disease patients: experience from a single Chinese center. Int Urol Nephrol. 2012; 44:549–556.23. Zhou X, Zhang L, Ji WJ, Yuan F, Guo ZZ, Pang B, Luo T, Liu X, Zhang WC, Jiang TM, et al. Variation in dietary salt intake induces coordinated dynamics of monocyte subsets and monocyte-platelet aggregates in humans: implications in end organ inflammation. PLoS One. 2013; 8:e60332.24. Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002; 61:579–585.25. Urushihara M, Kondo S, Kagami S, Kobori H. Urinary angiotensinogen accurately reflects intrarenal Renin-Angiotensin system activity. Am J Nephrol. 2010; 31:318–325.26. Kobori H, Alper AB Jr, Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, et al. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009; 53:344–350.27. Franco M, Martínez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodríguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2007; 293:R251–R256.28. Samuel P, Ali Q, Sabuhi R, Wu Y, Hussain T. High Na intake increases renal angiotensin II levels and reduces expression of the ACE2-AT(2)R-MasR axis in obese Zucker rats. Am J Physiol Renal Physiol. 2012; 303:F412–F419.29. Susic D, Frohlich ED, Kobori H, Shao W, Seth D, Navar LG. Salt-induced renal injury in SHRs is mediated by AT1 receptor activation. J Hypertens. 2011; 29:716–723.30. Mills KT, Kobori H, Hamm LL, Alper AB, Khan IE, Rahman M, Navar LG, Liu Y, Browne GM, Batuman V, et al. Increased urinary excretion of angiotensinogen is associated with risk of chronic kidney disease. Nephrol Dial Transplant. 2012; 27:3176–3181.31. Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol. 2003; 285:R117–R124.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes in urinary potassium excretion in patients with chronic kidney disease
- A Study on the Changes of Urinary Hormonal Excretion and Renal Function During Three-shift Nursing Practice
- Inhibition of indoxyl sulfate-induced intrarenal renin-angiotensin system activation: targeting the aryl hydrocarbon receptor
- Pathogenesis of diabetic nephropathy
- Altered Regulation of Renal Aquaporins and Sodium Transporters in Experimental Chronic Renal Failure