J Bacteriol Virol.
2007 Jun;37(2):91-103. 10.4167/jbv.2007.37.2.91.
Identification and Functional analysis of Gene Expression in Mycobacterium tuberculosis-infected Human Monocytic Cells Under Hypoxic Conditions
- Affiliations
-
- 11Department of Microbiology, College of Medicine, Chungnam National University, Daejeon 301-747, Korea. hayoungj@cnu.ac.kr
- 2Department of Microbiology, College of Medicine, Konyang University, Daejeon 302-718, Korea.
- 3Department of Internal Medicine, College of Medicine, Konyang University, Daejeon 302-718, Korea.
- KMID: 2055043
- DOI: http://doi.org/10.4167/jbv.2007.37.2.91
Abstract
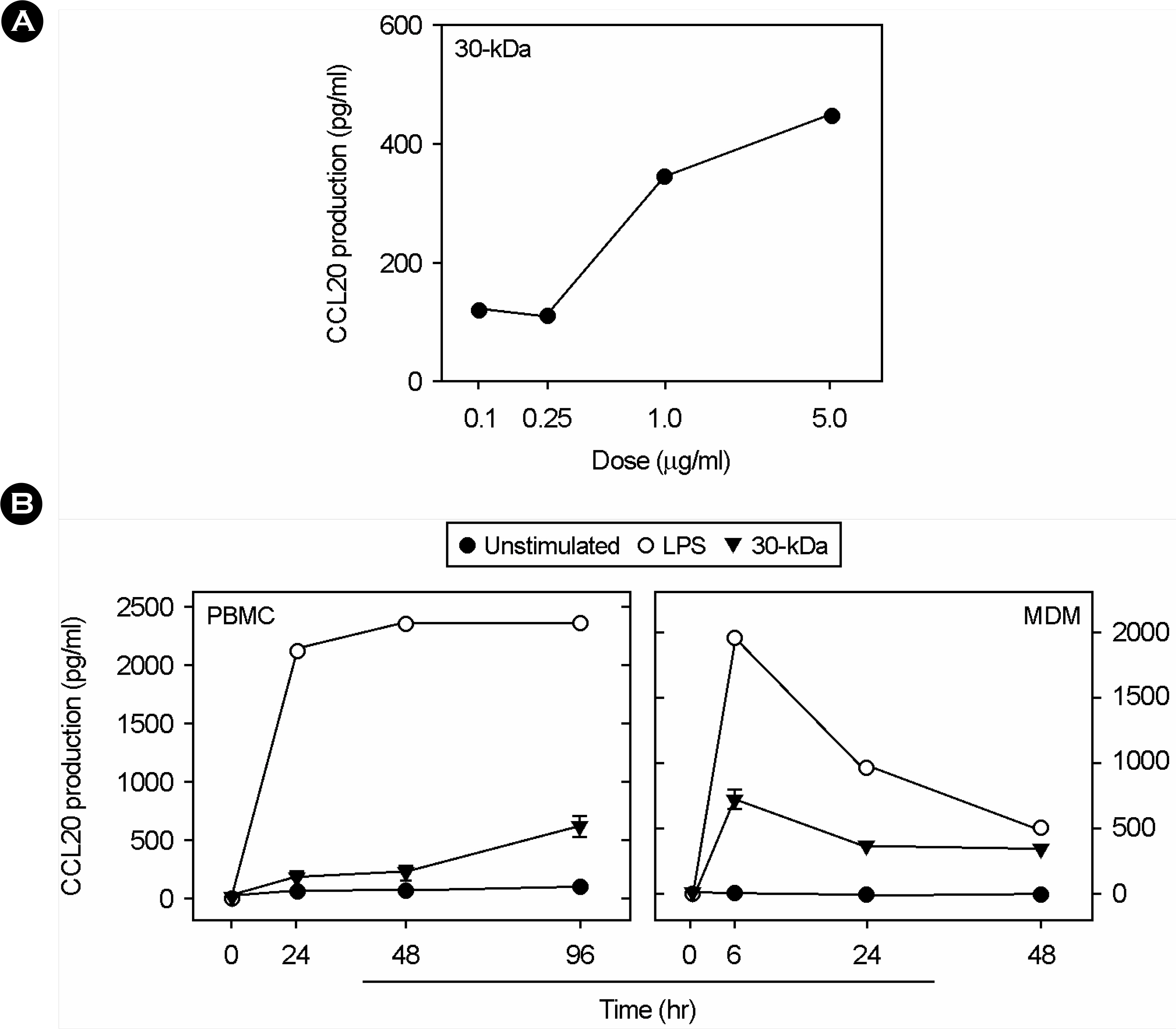
- Mycobacterium tuberculosis-induced granulomatous lesions, particularly those undergoing central caseation, are known as hypoxic. To analyze the host genes associated with hypoxic conditions from cells infected with M. tuberculosis, we performed GeneChip analyses on mRNA from M. tuberculosis H37Rv-treated human monocytic THP-1 cells cultured in oxygen-depleted status for 18 h. The expression of 99 genes was altered, including those involved in intracellular signaling, energy production, and protein metabolism, as revealed by stringent microarray data analysis. Most notably, mRNA expression of chemokine macrophage inflammatory protein 3alpha/CC chemokine ligand 20 (CCL20) was significantly up-regulated in M. tuberculosis-infected cells under hypoxic conditions. We further analyzed the CCL20 expression in peripheral blood mononuclear cells (PBMCs) and monocyte derived macrophages (MDMs) from healthy controls and TB patients. A comparative analysis has revealed that the mRNA and protein expression of CCL20 were prominently up-regulated in PBMCs, and MDMs from TB patients, compared with healthy controls. Collectively, these data show that the gene expression of CCL20 was up-regulated in M. tuberculosis H37Rv-infected human monocytic THP-1 cells cultured in hypoxic conditions. In addition, the production of CCL20 is substantially increased in cells from TB patients than in healthy controls, suggesting an important role of CCL20 in the immunopathogenesis during TB infection.
Keyword
MeSH Terms
Figure
Reference
-
References
1). Adams LB, Dinauer MC, Morgenstern DE, Krahenbuhl JL. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tuber Lung Dis. 78:237–246. 1997.2). Bosco MC, Puppo M, Santangelo C, Anfosso L, Pfeffer U, Fardin P, Battaglia F, Varesio L. Hypoxia modifies the transcriptome of primary human monocytes: modulation of novel immune-related genes and identification of CC-chemokine ligand 20 as a new hypoxia-inducible gene. J Immunol. 177:1941–1955. 2006.
Article3). Burke B, Giannoudis A, Corke KP, Gill D, Wells M, Ziegler-Heitbrock L, Lewis CE. Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am J Pathol. 163:1233–1243. 2003.4). Cazin M, Paluszezak D, Bianchi A, Cazin JC, Aerts C, Voisin C. Effects of anaerobiosis upon morphology and energy metabolism of alveolar macrophages cultured in gas phase. Eur Respir J. 3:1015–1022. 1990.5). Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, Sullivan LM, Smith SR, Greenberg HB, Narula SK, Lipp M, Lira SA. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 12:495–503. 2000.
Article6). Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 178:2243–2247. 1993.
Article7). Cooper AM, Segal BH, Frank AA, Holland SM, Orme IM. Transient loss of resistance to pulmonary tuberculosis in p47(phox–/–) mice. Infect Immun. 68:1231–1234. 2000.8). Demissie A, Abebe M, Aseffa A, Rook G, Fletcher H, Zumla A, Weldingh K, Brock I, Andersen P, Doherty TM. VACSEL Study Group: Healthy individuals that control a latent infection with Mycobacterium tuberculosis express high levels of Th1 cytokines and the IL-4 antagonist IL-4 delta2. J Immunol. 172:6938–6943. 2004.9). Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 188:373–386. 1998.
Article10). Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 282:677–686. 1999.11). Fenton MJ, Vermeulen MW. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect Immun. 64:683–690. 1996.
Article12). Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 19:93–129. 2001.
Article13). Flynn JL, Chan J. Tuberculosis: latency and reactivation. Infect Immun. 69:4195–4201. 2001.
Article14). Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 178:2249–2254. 1993.15). Greaves DR, Wang W, Dairaghi DJ, Dieu MC, Saint-Vis B, Franz-Bacon K, Rossi D, Caux C, McClanahan T, Gordon S, Zlotnik A, Schall TJ. CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3alpha and is highly expressed in human dendritic cells. J Exp Med. 186:837–844. 1997.16). Homey B, Dieu-Nosjean MC, Wiesenborn A, Massacrier C, Pin JJ, Oldham E, Catron D, Buchanan ME, Muller A, deWaal Malefyt R, Deng G, Orozco R, Ruzicka T, Lehmann P, Lebecque S, Caux C, Zlotnik A. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 164:6621–6632. 2000.17). Honer zu Bentrup K, Russell DG. Mycobacterial persistence: adaptation to a changing environment. Trends Microbiol. 9:597–605. 2001.18). Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, Tam L, Wu R. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J Immunol. 175:6676–6685. 2005.19). Kawaguchi T, Veech RL, Uyeda K. Regulation of energy metabolism in macrophages during hypoxia. Roles of fructose 2,6-bisphosphate and ribose 1,5-bisphosphate. J Biol Chem. 276:28554–28561. 2001.20). Lee JH, Kang HJ, Woo JS, Chae SW, Lee SH, Hwang SJ, Lee HM. Up-regulation of chemokine ligand 20 in chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg. 132:537–541. 2006.
Article21). Lee JS, Son JW, Jung SB, Kwon YM, Yang CS, Oh JH, Song CH, Kim HJ, Park JK, Paik TH, Jo EK. Ex vivo responses for interferon-gamma and proinflammatory cytokine secretion to low-molecular-weight antigen MTB12 of Mycobacterium tuberculosis during human tuberculosis. Scand J Immunol. 64:145–154. 2006.22). Lee JS, Song CH, Lim JH, Kim HJ, Park JK, Paik TH, Kim CH, Kong SJ, Shon MH, Jung SS, Jo EK. The production of tumour necrosis factor-alpha is decreased in peripheral blood mononuclear cells from multidrug-resistant tuberculosis patients following stimulation with the 30-kDa antigen of Mycobacterium tuberculosis. Clin Exp Immunol. 132:443–449. 2003.23). Parrish NM, Dick JD, Bishai WR. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 6:107–112. 1998.24). Rubie C, Frick VO, Wagner M, Rau B, Weber C, Kruse B, Kempf K, Tilton B, Konig J, Schilling M. Enhanced expression and clinical significance of CC-chemokine MIP-3 alpha in hepatocellular carcinoma. Scand J Immunol. 63:468–477. 2006.25). Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 14:409–426. 2003.
Article26). Sugita S, Kohno T, Yamamoto K, Imaizumi Y, Nakajima H, Ishimaru T, Matsuyama T. Induction of macrophage-inflammatory protein-3 alpha gene expression by TNF-α dependent NF-kappa B activation. J Immunol. 168:5621–5628. 2002.27). Song CH, Lee JS, Lee SH, Lim K, Kim HJ, Park JK, Paik TH, Jo EK. Role of mitogen-activated protein kinase pathways in the production of tumor necrosis factor-alpha, interleukin-10, and monocyte chemotactic protein-1 by Mycobacterium tuberculosis H37Rv-infected human monocytes. J Clin Immunol. 23:194–201. 2003.28). Ulrichs T, Kaufmann SH. New insights into the function of granulomas in human tuberculosis. J Pathol. 208:261–269. 2006.
Article29). Volpe E, Cappelli G, Grassi M, Martino A, Serafino A, Colizzi V, Sanarico N, Mariani F. Gene expression profiling of human macrophages at late time of infection with Mycobacterium tuberculosis. Immunology. 118:449–460. 2006.30). Wayne LG, Hayes LG. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun. 64:2062–2069. 1996.31). Werngren J, Hoffner SE. Drug-susceptible Mycobacterium tuberculosis Beijing genotype does not develop mutation-conferred resistance to rifampin at an elevated rate. J Clin Microbiol. 41:1520–1524. 2003.32). Yang D, Chen Q, Hoover DM, Staley P, Tucker KD, Lubkowski J, Oppenheim JJ. Many chemokines including CCL20/ MIP-3 alpha display antimicrobial activity. J Leukoc Biol. 74:448–455. 2003.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification and Functional Characterization of Differentially Expressed Genes in Human-derived Monocytic Cell Line U937 Infected with Mycobacterium tuberculosis H37Rv and Mycobacterium marinum: Comparative Evaluation of IL-8
- Up-regulation of prothymosin alpha in THP-1 cells infected with Mycobacterium tuberculosis
- Identification of Proteins Induced at Hypoxic and Low pH Conditions in Mycobacterium tuberculosis H37Rv
- Investigation of the Growth Rate Change in Recombinant BCG which was cloned Mycobacterium tuberculosis Adenylate Kinase Mutation Gene or Human Muscle-type Adenylate Kinase Synthetic Gene
- Identification of Mycobacterium tuberculosis Complex Using a Gene Probe method