Chonnam Med J.
2012 Dec;48(3):133-140. 10.4068/cmj.2012.48.3.133.
Antimicrobial Activity of Calcium Hydroxide in Endodontics: A Review
- Affiliations
-
- 1Department of Endodontics, School of Dentistry, Hamedan University of Medical Sciences, Hamedan, Iran. zahed_mohammadi@yahoo.com
- 2Iranian Center for Endodontic Research (ICER), Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 3General Dental Practitioner, Hamedan, Iran.
- 4Department of Endodontics, School of Dentistry, Ahvaz University of Medical Sciences, Ahvaz, Iran.
- KMID: 2048801
- DOI: http://doi.org/10.4068/cmj.2012.48.3.133
Abstract
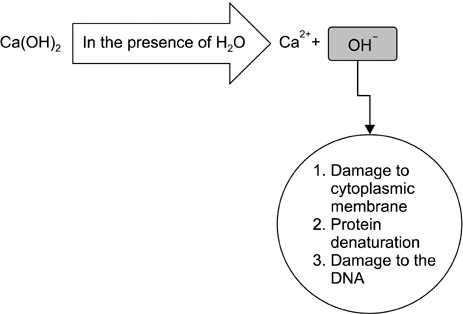
- The purpose of endodontic therapy is to preserve the patient's natural teeth without compromising the patient's local or systemic health. Calcium hydroxide has been included in several materials and antimicrobial formulations that are used in several treatment modalities in endodontics, such as inter-appointment intracanal medicaments. The purpose of this article was to review the antimicrobial properties of calcium hydroxide in endodontics. Calcium hydroxide has a high pH (approximately 12.5-12.8) and is classified chemically as a strong base. The lethal effects of calcium hydroxide on bacterial cells are probably due to protein denaturation and damage to DNA and cytoplasmic membranes. Calcium hydroxide has a wide range of antimicrobial activity against common endodontic pathogens but is less effective against Enterococcus faecalis and Candida albicans. Calcium hydroxide is also a valuable anti-endotoxin agent. However, its effect on microbial biofilms is controversial.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Antifungal effects of synthetic human β-defensin 3-C15 peptide
Sang-Min Lim, Ki-Bum Ahn, Christine Kim, Jong-Won Kum, Hiran Perinpanayagam, Yu Gu, Yeon-Jee Yoo, Seok Woo Chang, Seung Hyun Han, Won-Jun Shon, Woocheol Lee, Seung-Ho Baek, Qiang Zhu, Kee-Yeon Kum
Restor Dent Endod. 2016;41(2):91-97. doi: 10.5395/rde.2016.41.2.91.
Reference
-
1. Hermann BW. Calcium hydroxid als Mittelzurn, Behandeln und Fullen von Wurzelkanalen [Thesis]. 1920. Wurzburg:2. Farhad A, Mohammadi Z. Calcium hydroxide: a review. Int Dent J. 2005. 55:293–301.
Article3. Spångberg L, Haapasalo M. Rationale and efficacy of root canal medicaments and root filling materials with emphasis on treatment outcome. Endod Top. 2002. 2:35–58.
Article4. Rehman K, Saunders WP, Foye RH, Sharkey SW. Calcium ion diffusion from calcium hydroxide-containing materials in endodontically-treated teeth: an in vitro study. Int Endod J. 1996. 29:271–279.
Article5. Siqueira JF Jr. Strategies to treat infected root canals. J Calif Dent Assoc. 2001. 29:825–837.6. Siqueira JF Jr, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. 1999. 32:361–369.
Article7. Estrela C, Sydney GB, Bammann LL, Felippe O Jr. Estudo do efeito biológico do pH na atividade enzimática de bactérias anaeróbias. Rev Fac Odontol Bauru. 1994. 2:29–36.8. Estrela C, Pimenta FC, Ito IY, Bammann LL. Antimicrobial evaluation of calcium hydroxide in infected dentinal tubules. J Endod. 1999. 25:416–418.
Article9. Putnam RW. Intracellular pH regulation. Cell physiology. 1995. San Diego: Academic Press;212–229.10. Estrela C, Pécora JD, Silva RS. pH analysis of vehicles and calcium hydroxide pastes. Braz Endod J. 1998. 3:41–47.11. Bystrom A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985. 1:170–175.
Article12. Stevens RH, Grossman LI. Evaluation of the antimicrobial potential of calcium hydroxide as an intracanal medicament. J Endod. 1983. 9:372–374.
Article13. Sjögren U, Figdor D, Spångberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J. 1991. 24:119–125.
Article14. Han GY, Park SH, Yoon TC. Antimicrobial activity of Ca(OH)2 containing pastes with Enterococcus faecalis in vitro. J Endod. 2001. 27:328–332.
Article15. Estrela C, Rodrigues de Araújo Estrela C, Bammann LL, Pecora JD. Two methods to evaluate the antimicrobial action of calcium hydroxide paste. J Endod. 2001. 27:720–723.
Article16. Behnen MJ, West LA, Liewehr FR, Buxton TB, McPherson JC 3rd. Antimicrobial activity of several calcium hydroxide preparations in root canal dentin. J Endod. 2001. 27:765–767.
Article17. Lin S, Tsesis I, Zukerman O, Weiss EI, Fuss Z. Effect of electrophoretically activated calcium hydroxide on bacterial viability in dentinal tubules--in vitro. Dent Traumatol. 2005. 21:42–45.
Article18. Portenier I, Waltimo T, Ørstavik D, Haapasalo M. The susceptibility of starved, stationary phase, and growing cells of Enterococcus faecalis to endodontic medicaments. J Endod. 2005. 31:380–386.
Article19. DiFiore PM, Peters DD, Setterstrom JA, Lorton L. The antibacterial effects of calcium hydroxide apexification pastes on Streptococcus sanguis. Oral Surg Oral Med Oral Pathol. 1983. 55:91–94.
Article20. Siqueira JF Jr, Lopes HP, de Uzeda M. Recontamination of coronally unsealed root canals medicated with camphorated paramonochlorophenol or calcium hydroxide pastes after saliva challenge. J Endod. 1998. 24:11–14.
Article21. Haapasalo M, Orstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987. 66:1375–1379.22. Safavi KE, Spangberg LS, Langeland K. Root canal dentinal tubule disinfection. J Endod. 1990. 16:207–210.
Article23. Orstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod Dent Traumatol. 1990. 6:142–149.
Article24. Siqueira JF Jr, de Uzeda M. Disinfection by calcium hydroxide pastes of dentinal tubules infected with two obligate and one facultative anaerobic bacteria. J Endod. 1996. 22:674–676.
Article25. Weiger R, de Lucena J, Decker HE, Löst C. Vitality status of microorganisms in infected human root dentine. Int Endod J. 2002. 35:166–171.
Article26. Sathorn C, Parashos P, Messer H. Antibacterial efficacy of calcium hydroxide intracanal dressing: a systematic review and meta-analysis. Int Endod J. 2007. 40:2–10.
Article27. Cook J, Nandakumar R, Fouad AF. Molecular- and culture-based comparison of the effects of antimicrobial agents on bacterial survival in infected dentinal tubules. J Endod. 2007. 33:690–692.
Article28. Ballal V, Kundabala M, Acharya S, Ballal M. Antimicrobial action of calcium hydroxide, chlorhexidine and their combination on endodontic pathogens. Aust Dent J. 2007. 52:118–121.
Article29. Krithikadatta J, Indira R, Dorothykalyani AL. Disinfection of dentinal tubules with 2% chlorhexidine, 2% metronidazole, bioactive glass when compared with calcium hydroxide as intracanal medicaments. J Endod. 2007. 33:1473–1476.
Article30. Lee Y, Han SH, Hong SH, Lee JK, Ji H, Kum KY. Antimicrobial efficacy of a polymeric chlorhexidine release device using in vitro model of Enterococcus faecalis dentinal tubule infection. J Endod. 2008. 34:855–858.
Article31. Westphal O. Bacterial endotoxins. The second carl prausnitz memorial lecture. Int Arch Allergy Appl Immunol. 1975. 49:1–43.32. Rietschel ET, Brade H. Bacterial endotoxins. Sci Am. 1992. 267:54–61.
Article33. Leonardo MR, Silva RA, Assed S, Nelson-Filho P. Importance of bacterial endotoxin (LPS) in endodontics. J Appl Oral Sci. 2004. 12:93–98.
Article34. Barthel CR, Levin LG, Reisner HM, Trope M. TNF-alpha release in monocytes after exposure to calcium hydroxide treated Escherichia coli LPS. Int Endod J. 1997. 30:155–159.
Article35. Stashenko P. Role of immune cytokines in the pathogenesis of periapical lesions. Endod Dent Traumatol. 1990. 6:89–96.36. Yamasaki M, Nakane A, Kumazawa M, Hashioka K, Horiba N, Nakamura H. Endotoxin and gram-negative bacteria in the rat periapical lesions. J Endod. 1992. 18:501–504.
Article37. Leonardo MR, da Silva LA, Leonardo Rde T, Utrilla LS, Assed S. Histological evaluation of therapy using a calcium hydroxide dressing for teeth with incompletely formed apices and periapical lesions. J Endod. 1993. 19:348–352.
Article38. Katebzadeh N, Hupp J, Trope M. Histological periapical repair after obturation of infected root canals in dogs. J Endod. 1999. 25:364–368.
Article39. Nelson-Filho P, Leonardo MR, Silva LA, Assed S. Radiographic evaluation of the effect of endotoxin (LPS) plus calcium hydroxide on apical and periapical tissues of dogs. J Endod. 2002. 28:694–696.
Article40. Trope M, Delano EO, Orstavik D. Endodontic treatment of teeth with apical periodontitis: single vs. multivisit treatment. J Endod. 1999. 25:345–350.
Article41. Leonardo MR, Silva LAB, Leonardo RT. Feller C, Gorab R, editors. Tratamento de canal radicular em sessao unica: crenca vs. ciencia. Atualizacao na Clinica Odontologica. 2000. Sao Paulo: Artes Médicas;29–57.42. Safavi KE, Nichols FC. Effect of calcium hydroxide on bacterial lipopolysaccharide. J Endod. 1993. 19:76–78.
Article43. Safavi KE, Nichols FC. Alteration of biological properties of bacterial lipopolysaccharide by calcium hydroxide treatment. J Endod. 1994. 20:127–129.
Article44. Olsen MH, DiFiore PM, Dixit SN, Veis A. The effect of calcium hydroxide inhibition on LPS induced release of IL-1b from human monocytes in whole blood. J Endod. 1999. 25:289.
Article45. Silva L, Nelson-Filho P, Leonardo MR, Rossi MA, Pansani CA. Effect of calcium hydroxide on bacterial endotoxin in vivo. J Endod. 2002. 28:94–98.46. Tanomaru JM, Leonardo MR, Tanomaru Filho M, Bonetti Filho I, Silva LA. Effect of different irrigation solutions and calcium hydroxide on bacterial LPS. Int Endod J. 2003. 36:733–739.
Article47. Jiang J, Zuo J, Chen SH, Holliday LS. Calcium hydroxide reduces lipopolysaccharide-stimulated osteoclast formation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003. 95:348–354.
Article48. Buck RA, Cai J, Eleazer PD, Staat RH, Hurst HE. Detoxification of endotoxin by endodontic irrigants and calcium hydroxide. J Endod. 2001. 27:325–327.
Article49. Baumgartner JC, Watts CM, Xia T. Occurrence of Candida albicans in infections of endodontic origin. J Endod. 2000. 26:695–698.
Article50. Lana MA, Ribeiro-Sobrinho AP, Stehling R, Garcia GD, Silva BK, Hamdan JS, et al. Microorganisms isolated from root canals presenting necrotic pulp and their drug susceptibility in vitro. Oral Microbiol Immunol. 2001. 16:100–105.
Article51. Siqueira JF Jr, Sen BH. Fungi in endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004. 97:632–641.
Article52. Waltimo TMT, Haapasalo M, Zehnder M, Meyer J. Clinical aspects related to endodontic yeast infections. Endod Top. 2004. 9:66–78.
Article53. Waltimo TM, Orstavik D, Sirén EK, Haapasalo MP. In vitro susceptibility of Candida albicans to four disinfectants and their combinations. Int Endod J. 1999. 32:421–429.
Article54. Waltimo TM, Sirén EK, Orstavik D, Haapasalo MP. Susceptibility of oral Candida species to calcium hydroxide in vitro. Int Endod J. 1999. 32:94–98.
Article55. Siqueira JF Jr, Rôças IN, Magalhães FA, de Uzeda M. Antifungal effects of endodontic medicaments. Aust Endod J. 2001. 27:112–114.
Article56. Ferguson JW, Hatton JF, Gillespie MJ. Effectiveness of intracanal irrigants and medications against the yeast Candida albicans. J Endod. 2002. 28:68–71.
Article57. Valera MC, de Moraes Rego J, Jorge AO. Effect of sodium hypochlorite and five intracanal medications on Candida albicans in root canals. J Endod. 2001. 27:401–403.
Article58. Siqueira JF Jr, Rôças IN, Lopes HP, Magalhães FA, de Uzeda M. Elimination of Candida albicans infection of the radicular dentin by intracanal medications. J Endod. 2003. 29:501–504.59. Svensater G, Bergenholtz G. J Endod Biofilms in endodontic infections. Endod Top. 2004. 9:27–36.60. Ramachandran Nair PN. Light and electron microscopic studies of root canal flora and periapical lesions. J Endod. 1987. 13:29–39.
Article61. Nair PN, Henry S, Cano V, Vera J. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after "one-visit" endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005. 99:231–252.
Article62. Wu MK, Dummer PM, Wesselink PR. Consequences of and strategies to deal with residual post-treatment root canal infection. Int Endod J. 2006. 39:343–356.
Article63. Sen BH, Piskin B, Demirci T. Observation of bacteria and fungi in infected root canals and dentinal tubules by SEM. Endod Dent Traumatol. 1995. 11:6–9.
Article64. Gilbert P, Das J, Foley I. Biofilm susceptibility to antimicrobials. Adv Dent Res. 1997. 11:160–167.
Article65. Bowden GH, Hamilton IR. Survival of oral bacteria. Crit Rev Oral Biol Med. 1998. 9:54–85.
Article66. Distel JW, Hatton JF, Gillespie MJ. Biofilm formation in medicated root canals. J Endod. 2002. 28:689–693.
Article67. Chai WL, Hamimah H, Cheng SC, Sallam AA, Abdullah M. Susceptibility of Enterococcus faecalis biofilm to antibiotics and calcium hydroxide. J Oral Sci. 2007. 49:161–166.
Article68. Brändle N, Zehnder M, Weiger R, Waltimo T. Impact of growth conditions on susceptibility of five microbial species to alkaline stress. J Endod. 2008. 34:579–582.
Article69. Athanassiadis B, Abbott PV, Walsh LJ. The use of calcium hydroxide, antibiotics and biocides as antimicrobial medicaments in endodontics. Aust Dent J. 2007. 52:1 Suppl. S64–S82.
Article70. Mohammadi Z, Abbott PV. The properties and applications of chlorhexidine in endodontics. Int Endod J. 2009. 42:288–302.
Article71. Haenni S, Schmidlin PR, Mueller B, Sener B, Zehnder M. Chemical and antimicrobial properties of calcium hydroxide mixed with irrigating solutions. Int Endod J. 2003. 36:100–105.
Article72. Almyroudi A, Mackenzie D, McHugh S, Saunders WP. The effectiveness of various disinfectants used as endodontic intracanal medications: an in vitro study. J Endod. 2002. 28:163–167.
Article73. Gomes BP, Vianna ME, Sena NT, Zaia AA, Ferraz CC, de Souza Filho FJ. In vitro evaluation of the antimicrobial activity of calcium hydroxide combined with chlorhexidine gel used as intracanal medicament. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006. 102:544–550.74. Schäfer E, Bössmann K. Antimicrobial efficacy of chlorhexidine and two calcium hydroxide formulations against Enterococcus faecalis. J Endod. 2005. 31:53–56.75. Ercan E, Dalli M, Dülgergil CT. In vitro assessment of the effectiveness of chlorhexidine gel and calcium hydroxide paste with chlorhexidine against Enterococcus faecalis and Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006. 102:e27–e31.76. Onçag O, Cogulu D, Uzel A. Efficacy of various intracanal medicaments against Enterococcus faecalis in primary teeth: an in vivo study. J Clin Pediatr Dent. 2006. 30:233–237.77. Lin YH, Mickel AK, Chogle S. Effectiveness of selected materials against Enterococcus faecalis: part 3. The antibacterial effect of calcium hydroxide and chlorhexidine on Enterococcus faecalis. J Endod. 2003. 29:565–566.
Article78. Evans MD, Baumgartner JC, Khemaleelakul SU, Xia T. Efficacy of calcium hydroxide: chlorhexidine paste as an intracanal medication in bovine dentin. J Endod. 2003. 29:338–339.
Article79. Lindskog S, Pierce AM, Blomlöf L. Chlorhexidine as a root canal medicament for treating inflammatory lesions in the periodontal space. Endod Dent Traumatol. 1998. 14:186–190.
Article80. Haapasalo M, Qian W, Portenier I, Waltimo T. Effects of dentin on the antimicrobial properties of endodontic medicaments. J Endod. 2007. 33:917–925.
Article81. Haapasalo HK, Sirén EK, Waltimo TM, Ørstavik D, Haapasalo MP. Inactivation of local root canal medicaments by dentine: an in vitro study. Int Endod J. 2000. 33:126–131.
Article82. Portenier I, Haapasalo H, Rye A, Waltimo T, Ørstavik D, Haapasalo M. Inactivation of root canal medicaments by dentine, hydroxylapatite and bovine serum albumin. Int Endod J. 2001. 34:184–188.
Article83. Wang JD, Hume WR. Diffusion of hydrogen ion and hydroxyl ion from various sources through dentine. Int Endod J. 1988. 21:17–26.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antimicrobial effect of calcium hydroxide as an intracanal medicament in root canal treatment: a literature review - Part I. In vitro studies
- Antimicrobial effect of calcium hydroxide as an intracanal medicament in root canal treatment: a literature review - Part II. in vivo studies
- Effect of calcium hydroxide application time on dentin
- The significance of diagnosis and treatment planning in periapical lesion overfilled with calcium hydroxide paste
- Effect of calcium hydroxide on inflammatory root resorption and ankylosis in replanted teeth compared with other intracanal materials: a review