Anat Cell Biol.
2015 Mar;48(1):1-9. 10.5115/acb.2015.48.1.1.
When morphogenetic proteins encounter special extracellular matrix and cell-cell connections at the interface of the renal stem/progenitor cell niche
- Affiliations
-
- 1Institute of Molecular and Cellular Anatomy, University of Regensburg, Regensburg, Germany. will.minuth@vkl.uni-regensburg.de
- KMID: 2046069
- DOI: http://doi.org/10.5115/acb.2015.48.1.1
Abstract
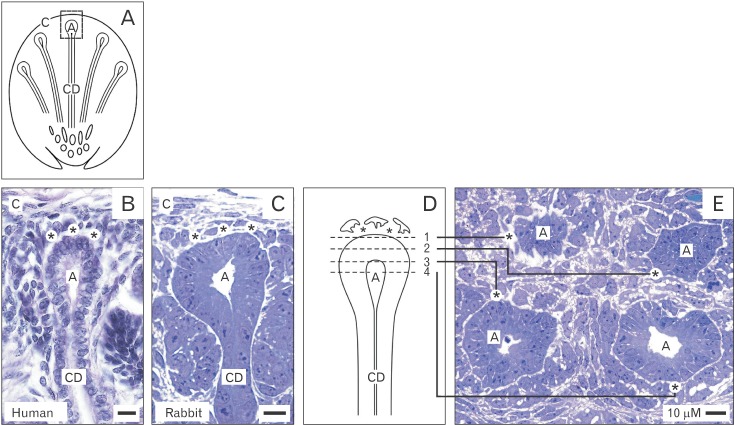
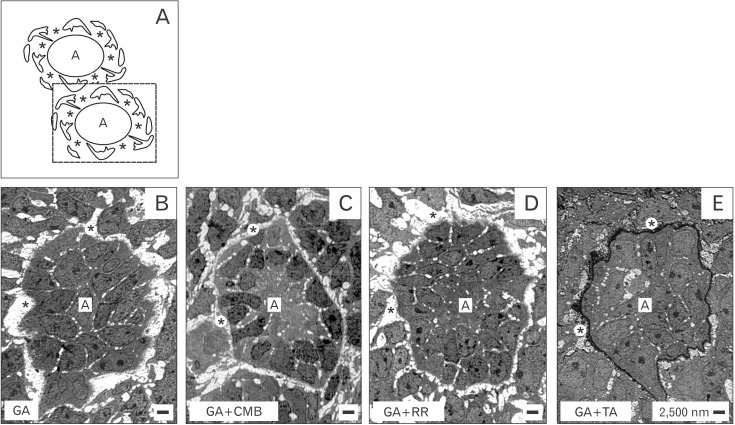
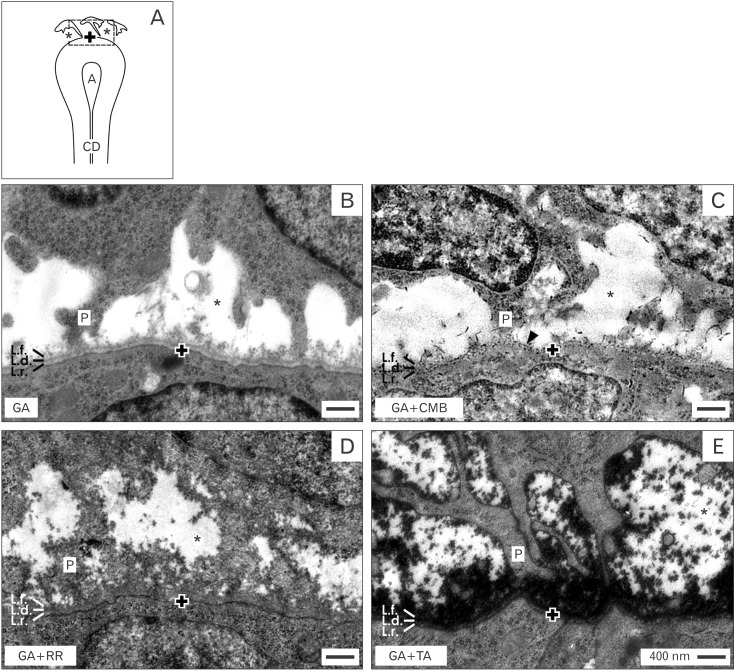
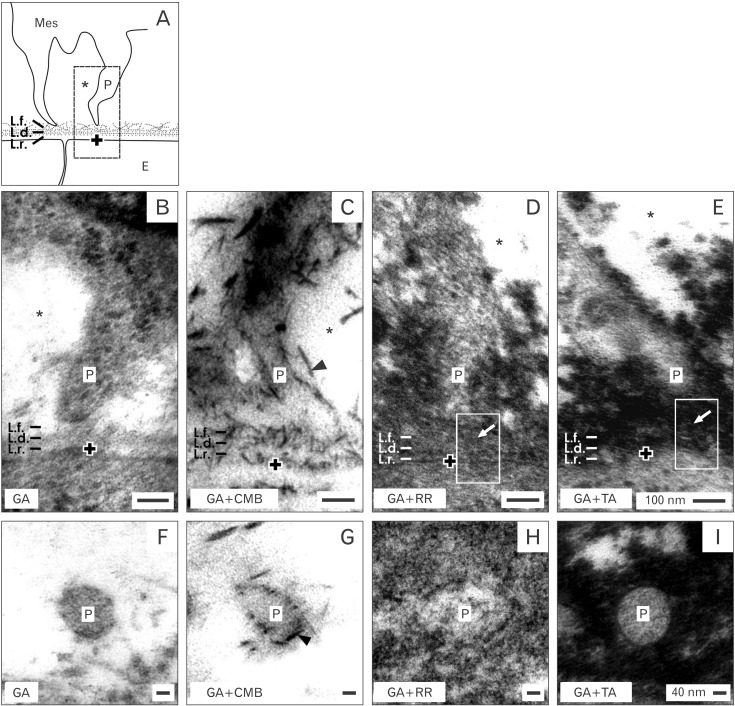
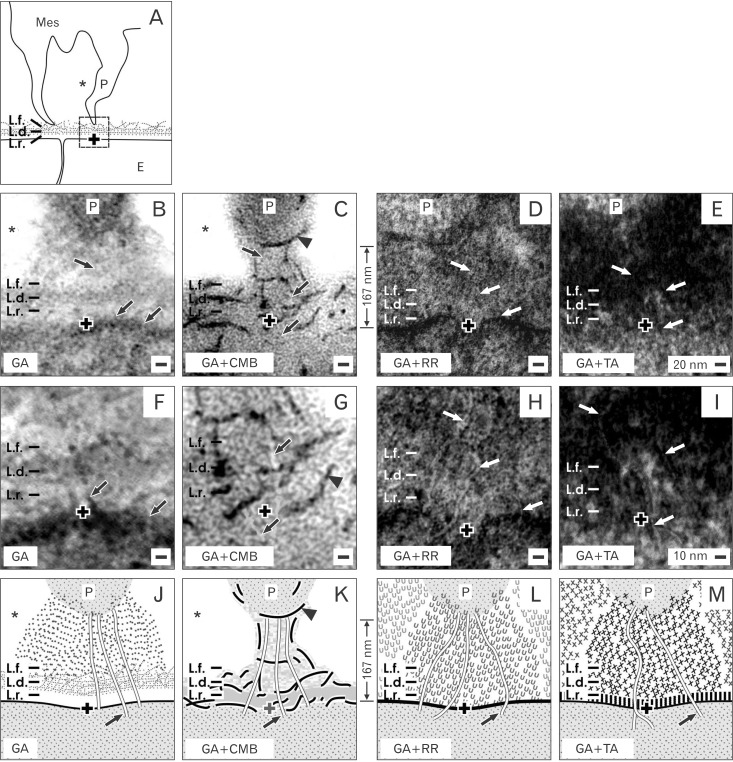
- Reciprocal exchange of morphogenetic proteins between epithelial and mesenchymal cells in a stem/progenitor cell niche results in formation of a nephron. To maintain diffusion of morphogenetic proteins, it is assumed that a close contact exists between involved cells. However, recent publications underline that both types of stem/progenitor cells are separated by a striking interface. To explore this microarchitecture in detail, neonatal rabbit kidneys were fixed in traditional glutaraldehyde (GA) solution for transmission electron microscopy. For contrast enhancing specimens were fixed in GA solution including cupromeronic blue, ruthenium red or tannic acid. To record same perspectives, embedded blocks of parenchyma were cut in exactly orientated vertical and transverse planes to lining collecting ducts. Electron microscopy of specimens fixed by traditional GA solution illustrates a spatial separation of stem/progenitor cells and an unobstrusively looking interface. In contrast, advanced fixation of specimens in GA solution including cupromeronic blue, ruthenium red and tannic acid unmasks earlier not visible extracellular matrix. In addition, projections of mesenchymal cells covered by matrix cross the interface to contact epithelial cells. Surprisingly, the end of a mesenchymal cell projection does not dangle but is enclosed in a fitting sleeve and connected via tunneling nanotubes with the plasma membrane of an epithelial cell. Regarding this complex ensemble the question is to what extent illustrated cell-cell connections and extracellular matrix are involved in communication and transmission of morphogenetic proteins during induction of a nephron.
MeSH Terms
Figure
Cited by 1 articles
-
The
in vitro analysis of migration and polarity of blastema cells in the extracellular matrix derived from bovine mesenteric in the presence of fibronectin
Kamelia Kohannezhad, Soroush Norouzi, Maryam Tafazoli, Safoura Soleymani, Nasser Mahdavi Shahri, Amin Tavassoli
Anat Cell Biol. 2022;55(2):229-238. doi: 10.5115/acb.21.233.
Reference
-
1. Meyer TN, Schwesinger C, Bush KT, Stuart RO, Rose DW, Shah MM, Vaughn DA, Steer DL, Nigam SK. Spatiotemporal regulation of morphogenetic molecules during in vitro branching of the isolated ureteric bud: toward a model of branching through budding in the developing kidney. Dev Biol. 2004; 275:44–67. PMID: 15464572.2. Datta A, Bryant DM, Mostov KE. Molecular regulation of lumen morphogenesis. Curr Biol. 2011; 21:R126–R136. PMID: 21300279.3. Blake J, Rosenblum ND. Renal branching morphogenesis: morphogenetic and signaling mechanisms. Semin Cell Dev Biol. 2014; 36:2–12. PMID: 25080023.4. Fanos V, Loddo C, Puddu M, Gerosa C, Fanni D, Ottonello G, Faa G. From ureteric bud to the first glomeruli: genes, mediators, kidney alterations. Int Urol Nephrol. 2015; 47:109–116. PMID: 25201458.5. Nishinakamura R. Stem cells in the embryonic kidney. Kidney Int. 2008; 73:913–917. PMID: 18200005.6. Kopan R, Chen S, Little M. Nephron progenitor cells: shifting the balance of self-renewal and differentiation. Curr Top Dev Biol. 2014; 107:293–331. PMID: 24439811.7. Minuth WW, Denk L. Structural links between the renal stem/progenitor cell niche and the organ capsule. Histochem Cell Biol. 2014; 141:459–471. PMID: 24429831.8. Carroll TJ, Das A. Defining the signals that constitute the nephron progenitor niche. J Am Soc Nephrol. 2013; 24:873–876. PMID: 23578945.9. Chai OH, Song CH, Park SK, Kim W, Cho ES. Molecular regulation of kidney development. Anat Cell Biol. 2013; 46:19–31. PMID: 23560233.10. O'Brien LL, McMahon AP. Induction and patterning of the metanephric nephron. Semin Cell Dev Biol. 2014; 36:31–38. PMID: 25194660.11. Kanwar YS, Wada J, Lin S, Danesh FR, Chugh SS, Yang Q, Banerjee T, Lomasney JW. Update of extracellular matrix, its receptors, and cell adhesion molecules in mammalian nephrogenesis. Am J Physiol Renal Physiol. 2004; 286:F202–F215. PMID: 14707006.12. Kim HY, Nelson CM. Extracellular matrix and cytoskeletal dynamics during branching morphogenesis. Organogenesis. 2012; 8:56–64. PMID: 22609561.13. Nigam SK, Bush KT. Growth factor-heparan sulfate "switches" regulating stages of branching morphogenesis. Pediatr Nephrol. 2014; 29:727–735. PMID: 24488503.14. Strehl R, Minuth WW. Partial identification of the mab (CD)Amp1 antigen at the epithelial-mesenchymal interface in the developing kidney. Histochem Cell Biol. 2001; 116:389–396. PMID: 11735003.15. Schumacher K, Strehl R, Minuth WW. Characterization of micro-fibers at the interface between the renal collecting duct ampulla and the cap condensate. Nephron Exp Nephrol. 2003; 95:e43–e54. PMID: 14610328.16. Schumacher K, Strehl R, De Vries U, Groene HJ, Minuth WW. SBA-positive fibers between the CD ampulla, mesenchyme, and renal capsule. J Am Soc Nephrol. 2002; 13:2446–2453. PMID: 12239233.17. Minuth WW, Denk L, Miess C, Glashauser A. Peculiarities of the extracellular matrix in the interstitium of the renal stem/progenitor cell niche. Histochem Cell Biol. 2011; 136:321–334. PMID: 21822715.18. Minuth WW, Denk L. Illustration of extensive extracellular matrix at the epithelial-mesenchymal interface within the renal stem/progenitor cell niche. BMC Clin Pathol. 2012; 12:16. PMID: 23009620.19. Minuth WW, Denk L. Advanced fixation for transmission electron microscopy unveils special extracellular matrix within the renal stem/progenitor cell niche. Methods Mol Biol. 2014; 7. 26. [Epub]. http://dx.doi.org/10.1007/7651_2014_93.20. Minuth WW, Denk L. Cell projections and extracellular matrix cross the interstitial interface within the renal stem/progenitor cell niche: accidental, structural or functional cues? Nephron Exp Nephrol. 2012; 122:131–140. PMID: 23735962.21. Muller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF. Integrin alpha8beta1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 1997; 88:603–613. PMID: 9054500.22. Brandenberger R, Schmidt A, Linton J, Wang D, Backus C, Denda S, Müller U, Reichardt LF. Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin alpha8beta1 in the embryonic kidney. J Cell Biol. 2001; 154:447–458. PMID: 11470831.23. Sato Y, Shimono C, Li S, Nakano I, Norioka N, Sugiura N, Kimata K, Yamada M, Sekiguchi K. Nephronectin binds to heparan sulfate proteoglycans via its MAM domain. Matrix Biol. 2013; 32:188–195. PMID: 23357641.24. Uchiyama Y, Sakaguchi M, Terabayashi T, Inenaga T, Inoue S, Kobayashi C, Oshima N, Kiyonari H, Nakagata N, Sato Y, Sekiguchi K, Miki H, Araki E, Fujimura S, Tanaka SS, Nishinakamura R. Kif26b, a kinesin family gene, regulates adhesion of the embryonic kidney mesenchyme. Proc Natl Acad Sci U S A. 2010; 107:9240–9245. PMID: 20439720.25. Nishinakamura R, Uchiyama Y, Sakaguchi M, Fujimura S. Nephron progenitors in the metanephric mesenchyme. Pediatr Nephrol. 2011; 26:1463–1467. PMID: 21336811.26. Kimura S, Hase K, Ohno H. The molecular basis of induction and formation of tunneling nanotubes. Cell Tissue Res. 2013; 352:67–76. PMID: 23229356.27. Austefjord MW, Gerdes HH, Wang X. Tunneling nanotubes: diversity in morphology and structure. Commun Integr Biol. 2014; 7:e27934. PMID: 24778759.28. Plotnikov EY, Khryapenkova TG, Galkina SI, Sukhikh GT, Zorov DB. Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Exp Cell Res. 2010; 316:2447–2455. PMID: 20599955.29. Domhan S, Ma L, Tai A, Anaya Z, Beheshti A, Zeier M, Hlatky L, Abdollahi A. Intercellular communication by exchange of cytoplasmic material via tunneling nano-tube like structures in primary human renal epithelial cells. PLoS One. 2011; 6:e21283. PMID: 21738629.30. Lander AD. Morpheus unbound: reimagining the morphogen gradient. Cell. 2007; 128:245–256. PMID: 17254964.31. Lehtonen E. Epithelio-mesenchymal interface during mouse kidney tubule induction in vivo. J Embryol Exp Morphol. 1975; 34:695–705. PMID: 1240119.32. Lehtonen E, Wartiovaara J, Nordling S, Saxén L. Demonstration of cytoplasmic processes in Millipore filters permitting kidney tubule induction. J Embryol Exp Morphol. 1975; 33:187–203. PMID: 1151265.33. Fleig SV, Humphreys BD. Rationale of mesenchymal stem cell therapy in kidney injury. Nephron Clin Pract. 2014; 127:75–80. PMID: 25343826.34. Herrera M, Mirotsou M. Stem cells: potential and challenges for kidney repair. Am J Physiol Renal Physiol. 2014; 306:F12–F23. PMID: 24197069.35. Burst V, Putsch F, Kubacki T, Völker LA, Bartram MP, Müller RU, Gillis M, Kurschat CE, Grundmann F, Müller-Ehmsen J, Benzing T, Teschner S. Survival and distribution of injected haematopoietic stem cells in acute kidney injury. Nephrol Dial Transplant. 2013; 28:1131–1139. PMID: 23197679.36. O'Neill JD, Freytes DO, Anandappa AJ, Oliver JA, Vunjak-Novakovic GV. The regulation of growth and metabolism of kidney stem cells with regional specificity using extracellular matrix derived from kidney. Biomaterials. 2013; 34:9830–9841. PMID: 24074840.37. Yu YL, Shao YK, Ding YQ, Lin KZ, Chen B, Zhang HZ, Zhao LN, Wang ZB, Zhang JS, Tang ML, Mei J. Decellularized kidney scaffold-mediated renal regeneration. Biomaterials. 2014; 35:6822–6828. PMID: 24855960.38. Tran C, Damaser MS. Stem cells as drug delivery methods: application of stem cell secretome for regeneration. Adv Drug Deliv Rev. 2014; 10. 15. [Epub]. http://dx.doi.org/10.1016/j.addr.2014.10.007.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem cell maintenance in a different niche
- Engineering the Extracellular Matrix for Organoid Culture
- Hormonal Regulation of Hematopoietic Stem Cells and Their Niche: A Focus on Estrogen
- Biomaterials Regulate Mechanosensors YAP/TAZ in Stem Cell Growth and Differentiation
- Regulation of the embryonic erythropoietic niche: a future perspective