J Korean Soc Magn Reson Med.
2011 Apr;15(1):22-31. 10.13104/jksmrm.2011.15.1.22.
Detection of Hepatic Lesion: Comparison of Free-Breathing and Respiratory-Triggered Diffusion-Weighted MR imaging on 1.5-T MR system
- Affiliations
-
- 1Department of Radiology, Ilsan Paik Hospital, Inje University College of Medicine, Korea. meditererian@hanmail.net
- KMID: 2041224
- DOI: http://doi.org/10.13104/jksmrm.2011.15.1.22
Abstract
- PURPOSE
To compare free-breathing and respiratory-triggered diffusion-weighted imaging on 1.5-T MR system in the detection of hepatic lesions.
MATERIALS AND METHODS
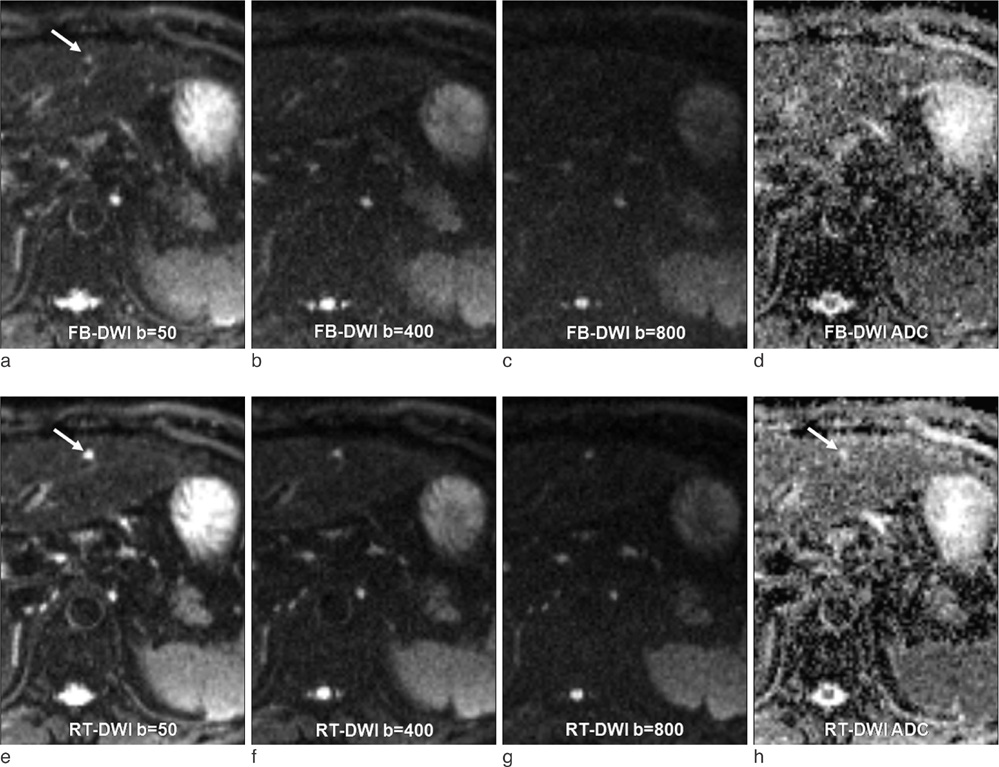
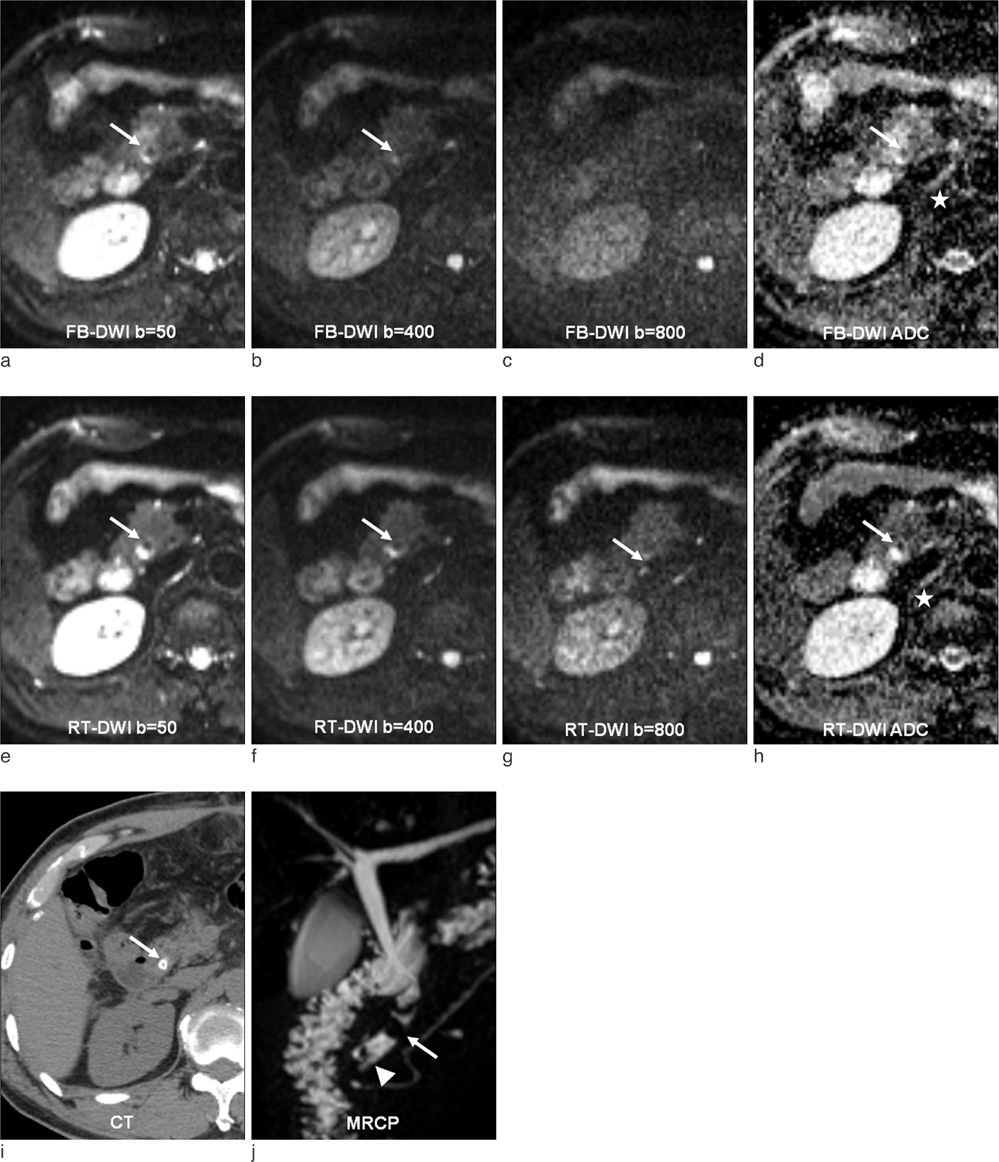
This single-institution study was approved by our institutional review board. Forty-seven patients (mean 57.9 year; M:F = 25:22) underwent hepatic MR imaging on 1.5-T MR system using both free-breathing and respiratory-triggered diffusion-weighted imaging (DWI) at a single examination. Two radiologists retrospectively reviewed respiratory-triggered and free-breathing sets (B50, B400, B800 diffusion weighted images and ADC map) in random order with a time interval of 2 weeks. Liver SNR and lesion-to-liver CNR of DWI were calculated measuring ROI.
RESULTS
Total of 62 lesions (53 benign, 9 malignant) that included 32 cysts, 13 hemangiomas, 7 hepatocellular carcinomas (HCCs), 5 eosinophilic infiltration, 2 metastases, 1 eosinophilic abscess, focal nodular hyperplasia, and pseudolipoma of Glisson's capsule were reviewed by two reviewers. Though not reaching statistical significance, the overall lesion sensitivities were increased in respiratory-triggered DWI [reviewer1: reviewer2, 47/62(75.81%):45/62(72.58%)] than free-breathing DWI [44/62(70.97%):41/62(66.13%)]. Especially for smaller than 1 cm hepatic lesions, sensitivity of respiratory-triggered DWI [24/30(80%):21/30(70%)] was superior to free-breathing DWI [17/30(56.7%):15/30(50%)]. The diagnostic accuracy measuring the area under the ROC curve (Az value) of free-breathing and respiratory-triggered DWI was not statistically different. Liver SNR and lesion-to-liver CNR of respiratorytriggered DWI (87.6+/-41.4, 41.2+/-62.5) were higher than free-breathing DWI (38.8+/-13.6, 24.8+/-36.8) (p value <0.001, respectively).
CONCLUSION
Respiratory-triggered diffusion-weighted MR imaging seemed to be better than free-breathing diffusion-weighted MR imaging on 1.5-T MR system for the detection of smaller than 1 cm lesions by providing high SNR and CNR.
MeSH Terms
Figure
Reference
-
1. Elsayes KM, Leyendecker JR, Menias CO, et al. MRI characterization of 124 CT-indeterminate focal hepatic lesions: evaluation of clinical utility. HPB (Oxford). 2007. 9:208–215.2. Ward J. New MR techniques for the detection of liver metastases. Cancer Imaging. 2006. 6:33–42.3. Muller MF, Prasad P, Siewert B, Nissenbaum MA, Raptopoulos V, Edelman RR. Abdominal diffusion mapping with use of a whole-body echo-planar system. Radiology. 1994. 190:475–478.4. Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with a single-shot echoplanar sequence: detection and characterization of focal hepatic lesions. AJR Am J Roentgenol. 1998. 170:397–402.5. Namimoto T, Yamashita Y, Sumi S, Tang Y, Takahashi M. Focal liver masses: characterization with diffusion-weighted echo-planar MR imaging. Radiology. 1997. 204:739–744.6. Vossen JA, Buijs M, Liapi E, Eng J, Bluemke DA, Kamel IR. Receiver operating characteristic analysis of diffusion-weighted magnetic resonance imaging in differentiating hepatic hemangioma from other hypervascular liver lesions. J Comput Assist Tomogr. 2008. 32:750–756.7. Bruegel M, Holzapfel K, Gaa J, et al. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur Radiol. 2008. 18:477–485.8. Kwee TC, Takahara T, Koh DM, Nievelstein RA, Luijten PR. Comparison and reproducibility of ADC measurements in breathhold, respiratory triggered, and free-breathing diffusion-weighted MR imaging of the liver. J Magn Reson Imaging. 2008. 28:1141–1148.9. Nasu K, Kuroki Y, Sekiguchi R, Nawano S. The effect of simultaneous use of respiratory triggering in diffusion-weighted imaging of the liver. Magn Reson Med Sci. 2006. 5:129–136.10. Gourtsoyianni S, Papanikolaou N, Yarmenitis S, Maris T, Karantanas A, Gourtsoyiannis N. Respiratory gated diffusion-weighted imaging of the liver: value of apparent diffusion coefficient measurements in the differentiation between most commonly encountered benign and malignant focal liver lesions. Eur Radiol. 2008. 18:486–492.11. Kandpal H, Sharma R, Madhusudhan KS, Kapoor KS. Respiratory-triggered versus breath-hold diffusion-weighted MRI of liver lesions: comparison of image quality and apparent diffusion coefficient values. AJR Am J Roentgenol. 2009. 192:915–922.12. Holzapfel K, Bruegel M, Eiber M, et al. Characterization of small (≤10mm) focal liver lesions: Value of respiratory-triggered echo-planar diffusion-weighted MR imaging. Eur J Radiol. 2009.13. Quan XY, Sun XJ, Yu ZJ, Tang M. Evaluation of diffusion weighted imaging of magnetic resonance imaging in small focal hepatic lesions: a quantitative study in 56 cases. Hepatobiliary Pancreat Dis Int. 2005. 4:406–409.14. Koh DM, Scurr E, Collins DJ, et al. Colorectal hepatic metastases: quantitative measurements using single-shot echo-planar diffusion-weighted MR imaging. Eur Radiol. 2006. 16:1898–1905.15. Nasu K, Kuroki Y, Nawano S, et al. Hepatic metastases: diffusion-weighted sensitivity-encoding versus SPIO-enhanced MR imaging. Radiology. 2006. 239:122–130.16. Xu PJ, Yan FH, Wang JH, Lin J, Ji Y. Added value of breathhold diffusion-weighted MRI in detection of small hepatocellular carcinoma lesions compared with dynamic contrast-enhanced MRI alone using receiver operating characteristic curve analysis. J Magn Reson Imaging. 2009. 29:341–349.17. Bruegel M, Gaa J, Waldt S, et al. Diagnosis of hepatic metastasis: comparison of respiration-triggered diffusionweighted echo-planar MRI and five t2-weighted turbo spin-echo sequences. AJR Am J Roentgenol. 2008. 191:1421–1429.18. Parikh T, Drew SJ, Lee VS, et al. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008. 246:812–822.19. Nasu K, Kuroki Y, Fujii H, Minami M. Hepatic pseudo-anisotropy: a specific artifact in hepatic diffusion-weighted images obtained with respiratory triggering. MAGMA. 2007. 20:205–211.20. Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001. 35:421–430.21. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of Hepatocellular carcinoma. Hepatology. 2005. 42:1208–1236.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MR Imaging of Skeletal Muscle Injury in Rabbit: Comparison bet ween Diffusion and T2-weighted MR Images
- Small Focal Hepatic Lesions <=1 cm: Their Detection and Characterization with Performing Diffusion-Weighted Sensitivity-Encoding versus SPIO-Enhanced 3T MR Imaging
- A Comparison of Lesion Detection and Conspicuity on T2-weighted Images (T2 FFE), FLAIR and Diffusion-weighted Images in Patients with Traumatic Brain Injury
- Comparison of Non-Breath-Hold T2-weighted Turbo Spin-Echo and Three Breath-Hold T2-weighted MR Images for Detection of Focal Hepatic Lesion
- Diffusion-Weighted MR Imaging in Biopsy-Proven Creutzfeldt-Jakob Disease