J Korean Soc Radiol.
2011 May;64(5):465-473. 10.3348/jksr.2011.64.5.465.
Diffusion-Weighted MR Imaging of Upper Abdomen: Comparison of Breath-Hold, Free-Breathing, and Respiratory-Triggered Techniques
- Affiliations
-
- 1Department of Radiology and the Research Institute of Radiological Science, Yonsei University College of Medicine, Gangnam Severance Hospital, Korea. yjsrad97@yuhs.ac
- KMID: 2040815
- DOI: http://doi.org/10.3348/jksr.2011.64.5.465
Abstract
- PURPOSE
To compare the image quality and stability of apparent diffusion coefficient (ADC) in diffusion-weighted MRI (DWI) of the upper abdomen among the breath-hold (BH), free-breathing (FB) and respiratory-triggered (RT) techniques.
MATERIALS AND METHODS
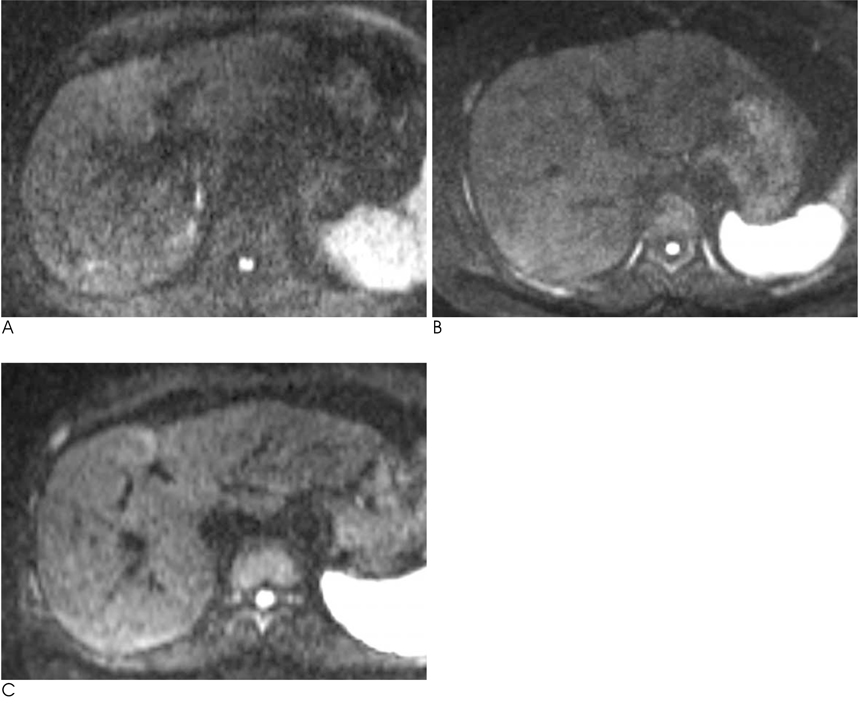
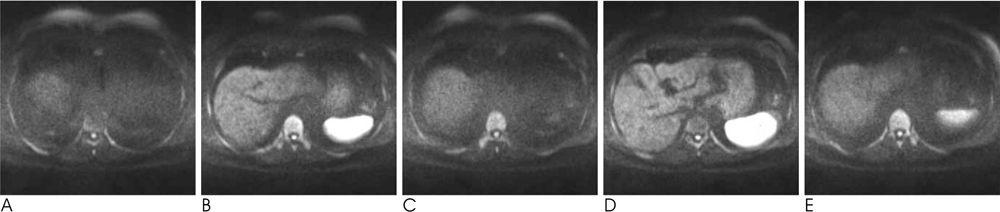
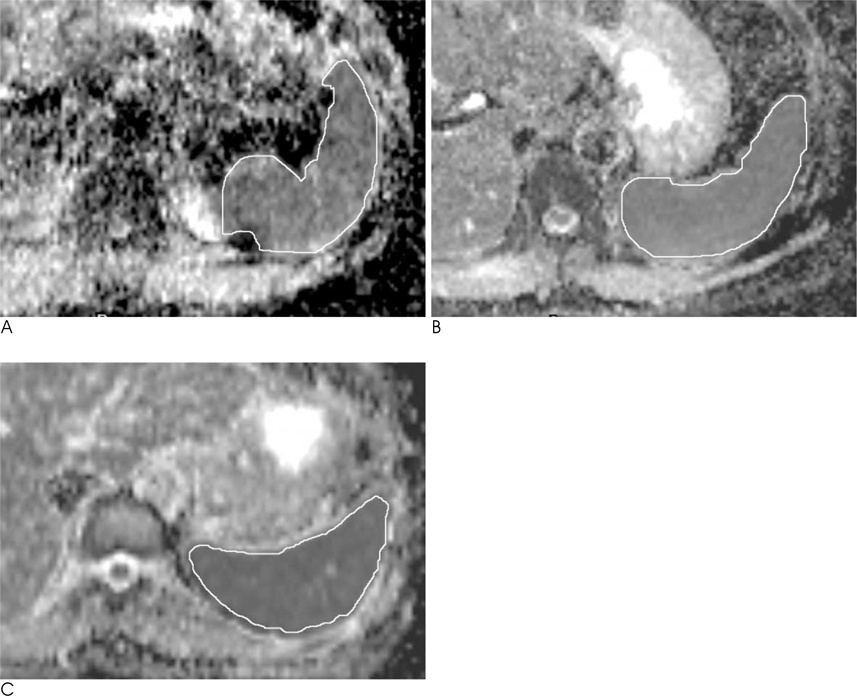
We analyzed the qualitative and quantitative parameters of 204 consecutive patients who underwent DWI (BH-DWI, FB-DWI or RT-DWI; n=68 in each technique). Qualitative parameters included liver contour, vascular landmarks, intra-slice homogeneity, and inter-slice discontinuity on DWI with a b-factor of 800 s/mm2 and a four-grade scale. Quantitative parameters included inter-slice or intraslice inhomogeneity of ADC in the spleen.
RESULTS
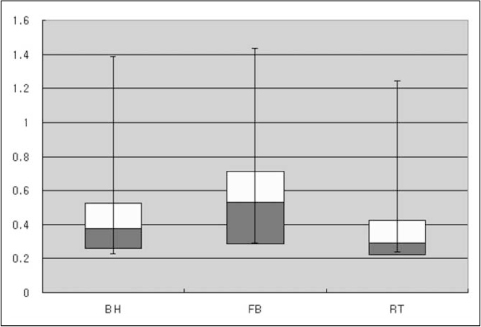
RT-DWI showed better liver contour compared to BH-DWI (p < 0.001) or FB-DWI (p = 0.001). As for the quality of the vascular landmarks, BH-DWI was inferior to FB-DWI (p = 0.025) and RT-DWI (p < 0.001). FB-DWI had the poorest result (p < 0.001) for inter-slice discontinuity compared to the other techniques. FB-DWI showed significantly larger inter-slice differences between the highest and the lowest ADCs in the spleen compared with those of RT-DWI (p < 0.001). Intra-slice homogeneity was significantly better in RT-DWI and FB-DWI than in BH-DWI (p < 0.001).
CONCLUSION
Compared with BH or FB techniques, RT-DWI appears to result in the best imaging by providing better anatomic detail without skipping continuous slices, in addition to more homogeneous ADCs.
MeSH Terms
Figure
Reference
-
1. Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with a single-shot echoplanar sequence: detection and characterization of focal hepatic lesions. AJR Am J Roentgenol. 1998; 170:397–402.2. Nasu K, Kuroki Y, Nawano S, et al. Hepatic metastases: diffusion-weighted sensitivity-encoding versus SPIO-enhanced MR imaging. Radiology. 2006; 239:122–130.3. Kim T, Murakami T, Takahashi S, Hori M, Tsuda K, Nakamura H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol. 1999; 173:393–398.4. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988; 168:497–505.5. Stejskal EO, Tanner JE. Spin diffusion measurements: spin-echo in the presence of a time dependent field gradient. J Chem Phys. 1962; 42:288–292.6. Naganawa S, Kawai H, Fukatsu H, Sakurai Y, Aoki I, Miura S, et al. Diffusion-weighted imaging of the liver: technical challenges and prospects for the future. Magn Reson Med Sci. 2005; 4:175–186.7. Kandpal H, Sharma R, Madhusudhan KS, Kapoor KS. Respiratory-triggered versus breath-hold diffusion-weighted MRI of liver lesions: comparison of image quality and apparent diffusion coefficient values. AJR Am J Roentgenol. 2009; 192:915–922.8. Kwee TC, Takahara T, Koh DM, Nievelstein RA, Luijten PR. Comparison and reproducibility of ADC measurements in breath-hold, respiratory triggered, and free-breathing diffusion-weighted MR imaging of the liver. J Magn Reson Imaging. 2008; 28:1141–1148.9. Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010; 254:47–66.10. Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007; 188:1622–1635.11. Charles-Edwards EM, deSouza NM. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging. 2006; 6:135–143.12. Koh DM, Takahara T, Imai Y, Collins DJ. Practical aspects of assessing tumors using clinical diffusion-weighted imaging in the body. Magn Reson Med Sci. 2007; 6:211–224.13. Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004; 22:275–282.14. Asbach P, Hein PA, Stemmer A, Wagner M, Hyppertz A, Hamm B, et al. Free-breathing echo-planar imaging based diffusion-weighted magnetic resonance imaging of the liver with prospective acquisition correction. J Comput Assist Tomogr. 2008; 32:372–378.15. Nasu K, Kuroki Y, Sekiguchi R, Nawano S. The effect of simultaneous use of respiratory triggering in diffusion-weighted imaging of the liver. Magn Reson Med Sci. 2006; 5:129–136.16. Nasu K, Kuroki Y, Fujii H, Minami M. Hepatic pseudo-anisotropy: a specific artifact in hepatic diffusion-weighted images obtained with respiratory triggering. MAGMA. 2007; 20:205–211.17. Kim SY, Lee SS, Byun JH, et al. Malignant hepatic tumors: short-term reproducibility of apparent diffusion coefficients with breath-hold and respiratory-triggered diffusion weighted MR imaging. Radiology. 2010; 255:815–823.18. Kim T, Murakami T, Takahashi S, Hori M, Tsuda K, Nakamura H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol. 1999; 173:393–398.19. Müller MF, Prasad P, Siewert B, Nissenbaum MA, Raptopoulos V, Edelman RR. Abdominal diffusion mapping with use of a whole-body echo-planar system. Radiology. 1994; 190:475–458.20. Weih KS, Driesel W, von Mengershausen M, Norris DG. Online motion correction for diffusion-weighted segmented-EPI and FLASH imaging. MAGMA. 2004; 16:277–283.21. Deng J, Omary RA, Larson AC. Multishot diffusion-weighted SPLICE PROPELLER MRI of the abdomen. Magn Reson Med. 2008; 59:947–953.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of Hepatic Lesion: Comparison of Free-Breathing and Respiratory-Triggered Diffusion-Weighted MR imaging on 1.5-T MR system
- MR Cholangiopancreatography: Comparison of Breath-hold Fast Spin Echo and Respiratory Triggered Fast Spin Echo Techniques
- Quasi-breath-hold (QBH) Biofeedback in Gated 3D Thoracic MRI: Feasibility Study
- Comparison of Non-Breath-Hold T2-weighted Turbo Spin-Echo and Three Breath-Hold T2-weighted MR Images for Detection of Focal Hepatic Lesion
- Optimal MR Pulse Sequences for Hepatic Hemangiomas: Comparison of T2-Weighted Turbo-Spin-Echo, T2-Weighted Breath-hold Turbo-Spin-Echo, and T1-Weighted FLASH Dynamic Imaging