Korean Circ J.
2011 Apr;41(4):209-212. 10.4070/kcj.2011.41.4.209.
A Case of a Senile Systemic Amyloidosis Patient Presenting With Angina Pectoris and Dilated Cardiomyopathy
- Affiliations
-
- 1Division of Cardiology, Cardiac and Vascular Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hcgwon@skku.edu
- KMID: 2028946
- DOI: http://doi.org/10.4070/kcj.2011.41.4.209
Abstract
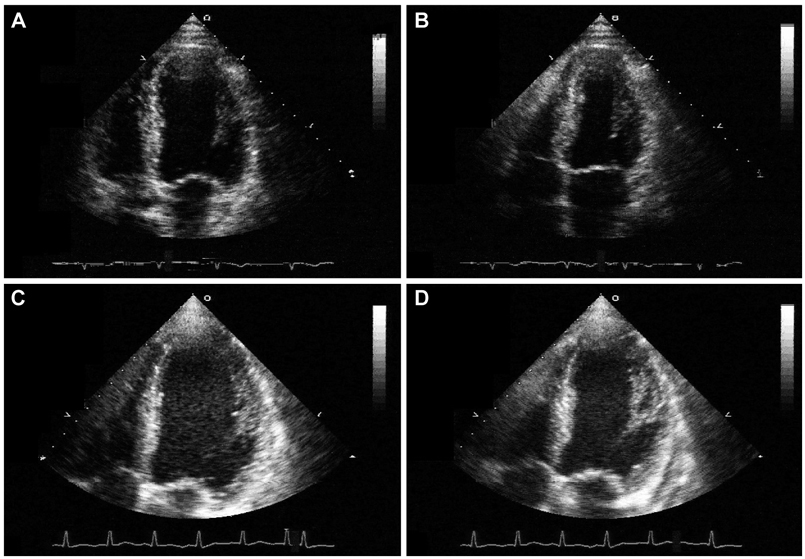
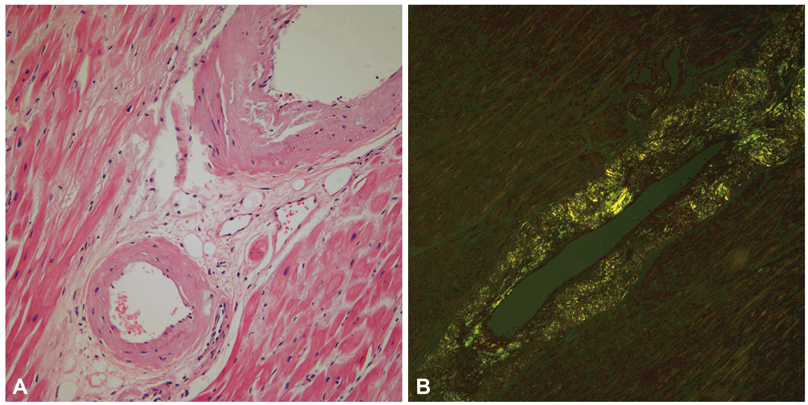
- A 77-year-old man visited our hospital complaining of aggravated exertional chest pain. He was diagnosed with syndrome X 7 years ago and underwent medical treatment in a regional hospital. Coronary angiography and echocardiography did not show any significant abnormalities. On the seventh in-hospital day, cardiogenic shock developed and echocardiography showed a dilated left ventricular (LV) cavity and severe LV systolic dysfunction. We thus inserted an intra-aortic balloon pump for hemodynamic support and were forced to maintain it because of weaning failure several times. Finally, heart transplantation was the decided necessary procedure. After successful heart transplantation, the biopsy specimen revealed a wild-type transthyretin deposition indicating senile systemic amyloidosis in the intramuscular coronary vessels and interstitium. Cardiac biopsy at the 4-year follow-up showed no recurrence of amyloid deposition.
MeSH Terms
Figure
Reference
-
1. McCarthy RE 3rd, Kasper EK. A review of the amyloidoses that infiltrate the heart. Clin Cardiol. 1998. 21:547–552.2. Cornwell GG 3rd, Westermark P. Senile amyloidosis: a protean manifestation of the aging process. J Clin Pathol. 1980. 33:1146–1152.3. Al Suwaidi J, Velianou JL, Gertz MA, et al. Systemic amyloidosis presenting with angina pectoris. Ann Intern Med. 1999. 131:838–841.4. Whitaker DC, Tungekar MF, Dussek JE. Angina with a normal coronary angiogram caused by amyloidosis. Heart. 2004. 90:e54.5. Mueller PS, Edwards WD, Gertz MA. Symptomatic ischemic heart disease resulting from obstructive intramural coronary amyloidosis. Am J Med. 2000. 109:181–188.6. Yamano S, Motomiya K, Akai Y, et al. Primary systemic amyloidosis presenting as angina pectoris due to intramyocardial coronary artery involvement: a case report. Heart Vessels. 2002. 16:157–160.7. Zabernigg A, Schranzhofer R, Kreczy A, Gattringer K. Continuously elevated cardiac troponin I in two patients with multiple myeloma and fatal cardiac amyloidosis. Ann Oncol. 2003. 14:1791.8. Cantwell RV, Aviles RJ, Bjornsson J, et al. Cardiac amyloidosis presenting with elevations of cardiac troponin I and angina pectoris. Clin Cardiol. 2002. 25:33–37.9. Kaski JC, Elliott PM. Angina pectoris and normal coronary arteriograms: clinical presentation and hemodynamic characteristics. Am J Cardiol. 1995. 76:35D–42D.10. Ogawa H, Mizuno Y, Ohkawara S, et al. Cardiac amyloidosis presenting as microvascular angina: a case report. Angiology. 2001. 52:273–278.11. Mesquita T, Chorao M, Soares I, Mello e Silva A, Abecasis P. Primary amyloidosis as a cause of microvascular angina and intermittent claudication. Rev Port Cardiol. 2005. 24:1521–1531.12. Ikeda S. Unusual clinical manifestations of primary systemic AL amyloidosis: are myasthenic symptoms and dilated cardiomyopathy caused by muscular or myocardial amyloid angiopathy? Intern Med. 2006. 45:123–124.13. Yoshita M, Ishida C, Yanase D, Yamada M. Immunoglobulin light-chain (AL) amyloidosis with myasthenic symptoms and echocardiographic features of dilated cardiomyopathy. Intern Med. 2006. 45:159–162.14. Tsuda T, Izumi T, Shibata A. Clinical assessment of serum myosin light chain I in patients with dilated cardiomyopathy. Kaku Igaku. 1992. 29:1035–1039.15. Siqueira-Filho AG, Cunha CL, Tajik AJ, Seward JB, Schattenberg TT, Giuliani ER. M-mode and two-dimensional echocardiographic features in cardiac amyloidosis. Circulation. 1981. 63:188–196.16. Chae JK. A case of secondary amyloidosis involving heart. Korean Circ J. 2000. 30:1179–1183.17. Oh SS, Youn HJ, Park JH, et al. Endomyocardial biopsy: one center's report about its role. Korean Circ J. 2008. 38:374–378.18. Fuchs U, Zittermann A, Suhr O, et al. Heart transplantation in a 68-year-old patient with senile systemic amyloidosis. Am J Transplant. 2005. 5:1159–1162.19. Dubrey SW, Burke MM, Hawkins PN, Banner NR. Cardiac transplantation for amyloid heart disease: the United Kingdom experience. J Heart Lung Transplant. 2004. 23:1142–1153.20. Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005. 112:2047–2060.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Polycythemia Vera Presenting with Angina Pectoris without Coronary Artery Stenosis
- A Case of Pheochromocytoma Presenting as Stress-Induced Cardiomyopathy with Large Left Ventricular Thrombus
- A Case of Apical Hypertrohic Cardiomyopathy Associated with Pheochromocytoma
- Diagnosis of angina pectoris
- A Case of Pheochromocytoma Presented with Cardiogenic Shock