J Periodontal Implant Sci.
2014 Oct;44(5):251-258. 10.5051/jpis.2014.44.5.251.
Tomographic and histometric analysis of autogenous bone block and synthetic hydroxyapatite block grafts without rigid fixation on rabbit calvaria
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. drjew@yuhs.ac
- 2Department of Periodontology, Dankook University College of Dentistry, Cheonan, Korea.
- 3Research Center for Oral Disease Regulation of the Aged, Chosun University School of Dentistry, Gwangju, Korea.
- KMID: 2027823
- DOI: http://doi.org/10.5051/jpis.2014.44.5.251
Abstract
- PURPOSE
The preferred material for bone augmentation beyond the envelope of skeletal bone is the bone block graft, due to its dimensional stability. We evaluated the necessity of rigid fixation for the bone block graft, and compared the bone regeneration and volume maintenance associated with grafting using a synthetic hydroxyapatite block (HAB) and an autogenous bone block (ABB) without rigid fixation on rabbit calvaria over two different periods.
METHODS
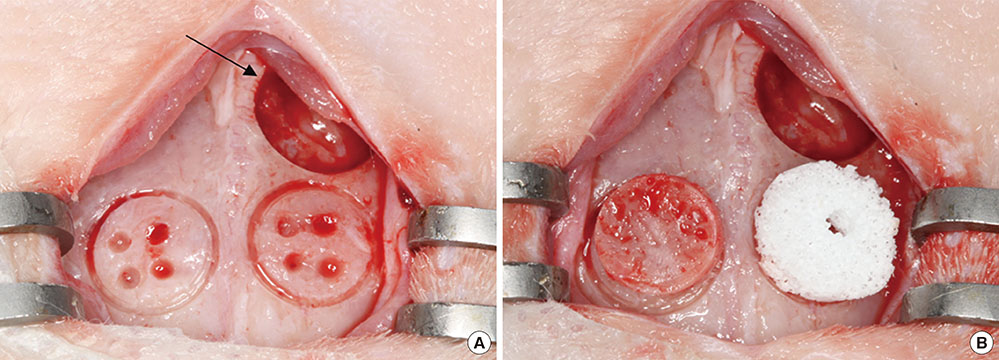
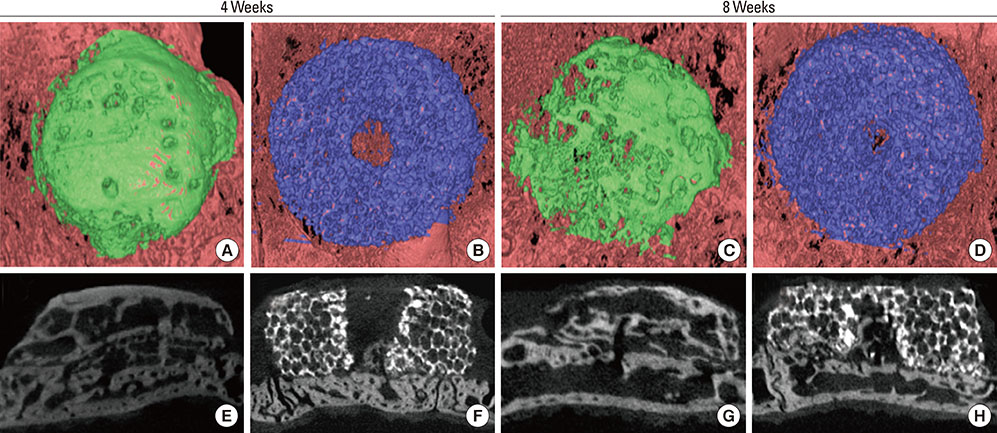
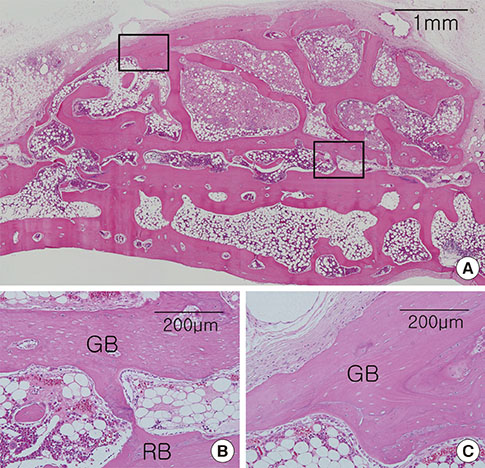
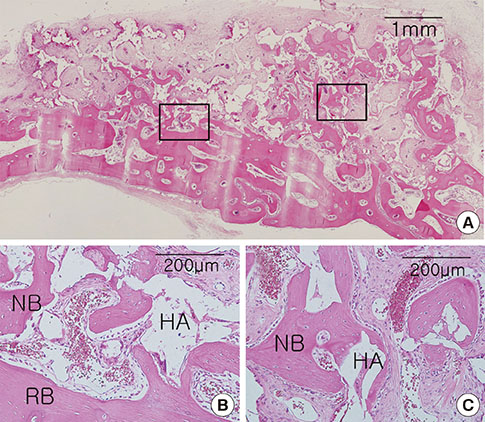
Cylinder-shaped synthetic HAB and ABB were positioned without fixation on the rabbit calvarium (n=16). The animals were sacrificed at 4 or 8 weeks postoperatively, and the grafted materials were analyzed at each healing period using microcomputed tomography and histologic evaluation.
RESULTS
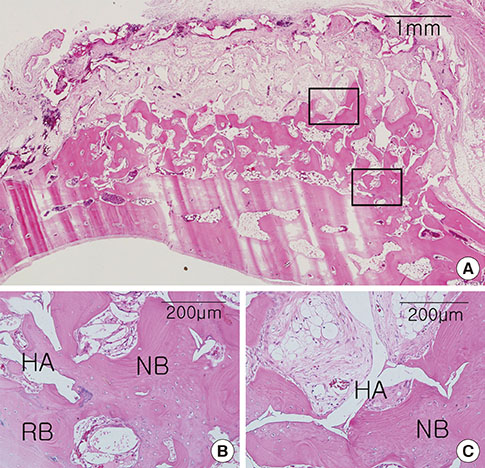
Integration of the graft and the recipient bed was observed in all specimens, although minor dislocation of the graft materials from the original position was evident in some specimens (six ABB and ten HAB samples). A tendency toward progressive bone resorption was observed in the grafted ABB but not in the grafted HAB, which maintained an intact appearance. In the HAB group, the area of new bone increased between 4 and 8 weeks postoperatively, but the difference was not statistically significant.
CONCLUSIONS
The nonfixed HAB was successfully integrated into the recipient bed after both healing periods in the rabbit calvaria. In spite of limited bone formation activity in comparison to ABB, HAB may be a favorable substitute osteoconductive bone material.
MeSH Terms
Figure
Reference
-
1. McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007; 78:377–396.
Article2. Fiorellini JP, Nevins ML. Localized ridge augmentation/preservation: a systematic review. Ann Periodontol. 2003; 8:321–327.3. Wang HL, Boyapati L. "PASS" principles for predictable bone regeneration. Implant Dent. 2006; 15:8–17.
Article4. Barbosa DZ, de Assis WF, Shirato FB, Moura CC, Silva CJ, Dechichi P. Autogenous bone graft with or without perforation of the receptor bed: histologic study in rabbit calvaria. Int J Oral Maxillofac Implants. 2009; 24:463–468.5. Jardini MA, De Marco AC, Lima LA. Early healing pattern of autogenous bone grafts with and without e-PTFE membranes: a histomorphometric study in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005; 100:666–673.
Article6. Hoexter DL. Bone regeneration graft materials. J Oral Implantol. 2002; 28:290–294.
Article7. Nishimura I, Shimizu Y, Ooya K. Effects of cortical bone perforation on experimental guided bone regeneration. Clin Oral Implants Res. 2004; 15:293–300.
Article8. Cha JK, Kim CS, Choi SH, Cho KS, Chai JK, Jung UW. The influence of perforating the autogenous block bone and the recipient bed in dogs. Part II: histologic analysis. Clin Oral Implants Res. 2012; 23:987–992.
Article9. Oh KC, Cha JK, Kim CS, Choi SH, Chai JK, Jung UW. The influence of perforating the autogenous block bone and the recipient bed in dogs. Part I: a radiographic analysis. Clin Oral Implants Res. 2011; 22:1298–1302.
Article10. Pinholt EM, Solheim E, Talsnes O, Larsen TB, Bang G, Kirkeby OJ. Revascularization of calvarial, mandibular, tibial, and iliac bone grafts in rats. Ann Plast Surg. 1994; 33:193–197.
Article11. Lin KY, Bartlett SP, Yaremchuk MJ, Fallon M, Grossman RF, Whitaker LA. The effect of rigid fixation on the survival of onlay bone grafts: an experimental study. Plast Reconstr Surg. 1990; 86:449–456.12. Zins JE, Whitaker LA. Membranous versus endochondral bone: implications for craniofacial reconstruction. Plast Reconstr Surg. 1983; 72:778–785.13. Phillips JH, Rahn BA. Fixation effects on membranous and endochondral onlay bone-graft resorption. Plast Reconstr Surg. 1988; 82:872–877.
Article14. Phillips JH, Rahn BA. Fixation effects on membranous and endochondral onlay bone graft revascularization and bone deposition. Plast Reconstr Surg. 1990; 85:891–897.
Article15. LaTrenta GS, McCarthy JG, Breitbart AS, May M, Sissons HA. The role of rigid skeletal fixation in bone-graft augmentation of the craniofacial skeleton. Plast Reconstr Surg. 1989; 84:578–588.
Article16. Burchardt H. The biology of bone graft repair. Clin Orthop Relat Res. 1983; (174):28–42.
Article17. De Marco AC, Jardini MA, Lima LP. Revascularization of autogenous block grafts with or without an e-PTFE membrane. Int J Oral Maxillofac Implants. 2005; 20:867–874.18. Jang YJ, Jung IH, Park JC, Jung UW, Kim CS, Lee YK, et al. Effect of seeding using an avidin-biotin binding system on the attachment of periodontal ligament fibroblasts to nanohydroxyapatite scaffolds: three-dimensional culture. J Periodontal Implant Sci. 2011; 41:73–78.
Article19. Kim MC, Lee BH, Kim KN, Kim KM, Choi SH, Kim CK, et al. Application of X-ray micro-computed tomography on macroporous calcium phosphate glass scaffolds. Key Eng Mater. 2006; 309-311:1087–1090.
Article20. Gosain AK, Song L, Yu P, Mehrara BJ, Maeda CY, Gold LI, et al. Osteogenesis in cranial defects: reassessment of the concept of critical size and the expression of TGF-beta isoforms. Plast Reconstr Surg. 2000; 106:360–371.
Article21. Frame JW. A convenient animal model for testing bone substitute materials. J Oral Surg. 1980; 38:176–180.22. Sohn JY, Park JC, Um YJ, Jung UW, Kim CS, Cho KS, et al. Spontaneous healing capacity of rabbit cranial defects of various sizes. J Periodontal Implant Sci. 2010; 40:180–187.
Article23. Mercier P, Bellavance F, Cholewa J, Djokovic S. Long-term stability of atrophic ridges reconstructed with hydroxylapatite: a prospective study. J Oral Maxillofac Surg. 1996; 54:960–968.
Article24. el Deeb M, Tompach PC, Morstad AT, Kwon P. Long-term follow-up of the use of nonporous hydroxyapatite for augmentation of the alveolar ridge. J Oral Maxillofac Surg. 1991; 49:257–261.
Article25. Proussaefs P, Lozada J, Valencia G, Rohrer MD. Histologic evaluation of a hydroxyapatite onlay bone graft retrieved after 9 years: a clinical report. J Prosthet Dent. 2002; 87:481–484.
Article26. Gao H, Tan T, Wang D. Effect of composition on the release kinetics of phosphate controlled release glasses in aqueous medium. J Control Release. 2004; 96:21–28.
Article27. Jammet P, Souyris F, Baldet P, Bonnel F, Huguet M. The effect of different porosities in coral implants: an experimental study. J Craniomaxillofac Surg. 1994; 22:103–108.
Article28. Leeuwenburgh S, Layrolle P, Barrere F, de Bruijn J, Schoonman J, van Blitterswijk CA, et al. Osteoclastic resorption of biomimetic calcium phosphate coatings in vitro. J Biomed Mater Res. 2001; 56:208–215.
Article29. Donath K, Rohrer MD, Beck-Mannagetta J. A histologic evaluation of a mandibular cross section one year after augmentation with hydroxyapatite particles. Oral Surg Oral Med Oral Pathol. 1987; 63:651–655.
Article30. Goto T, Kojima T, Iijima T, Yokokura S, Kawano H, Yamamoto A, et al. Resorption of synthetic porous hydroxyapatite and replacement by newly formed bone. J Orthop Sci. 2001; 6:444–447.
Article31. Jang JW, Yun JH, Lee KI, Jang JW, Jung UW, Kim CS, et al. Osteoinductive activity of biphasic calcium phosphate with different rhBMP-2 doses in rats. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 113:480–487.
Article32. Kim JW, Choi KH, Yun JH, Jung UW, Kim CS, Choi SH, et al. Bone formation of block and particulated biphasic calcium phosphate lyophilized with Escherichia coli-derived recombinant human bone morphogenetic protein 2 in rat calvarial defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 112:298–306.
Article33. Park JC, So SS, Jung IH, Yun JH, Choi SH, Cho KS, et al. Induction of bone formation by Escherichia coli-expressed recombinant human bone morphogenetic protein-2 using block-type macroporous biphasic calcium phosphate in orthotopic and ectopic rat models. J Periodontal Res. 2011; 46:682–690.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vertical Ridge Augmentation with Mandibular Ramus Block Bone for Implant Surgery
- Clinical Study on the Alveolar Bone Repair Capacity of Dentin Matrix Block
- Reconstruction of Mandibular Bone Defect Using a Titanium Mesh with Autogenous Particulate Cortical Bone Graft by an Intraoral Approach: A Case Report
- Revision Arthroplasty in Acetabular Defect
- Periimplant Bone Regeneration in Hydroxyapatite Block Grafts with Mesenchymal Stem Cells and Bone Morphogenetic Protein-2