Korean J Physiol Pharmacol.
2012 Aug;16(4):273-280. 10.4196/kjpp.2012.16.4.273.
A Simple Dosing Scheme for Intravenous Busulfan Based on Retrospective Population Pharmacokinetic Analysis in Korean Patients
- Affiliations
-
- 1Department of Clinical Pharmacology & Therapeutics, University of Ulsan College of Medicine, Asan Medical Center, Seoul 138-736, Korea. ksbae@amc.seoul.kr
- 2Division of Clinical Pharmacology, Clinical Trials Center, Pusan National University Hospital, Busan 602-739, Korea.
- 3Institute of Metabolism, Green Cross Laboratories, Yongin 446-850, Korea.
- 4Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul 110-744, Korea.
- 5Department of Clinical Pharmacology, Inje University Busan Paik Hospital, Busan 614-735, Korea.
- 6Department of Clinical Pharmacology and Therapeutics, Samsung Medical Center, Seoul 135-710, Korea.
- KMID: 2011162
- DOI: http://doi.org/10.4196/kjpp.2012.16.4.273
Abstract
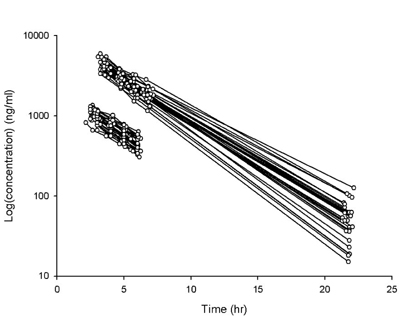
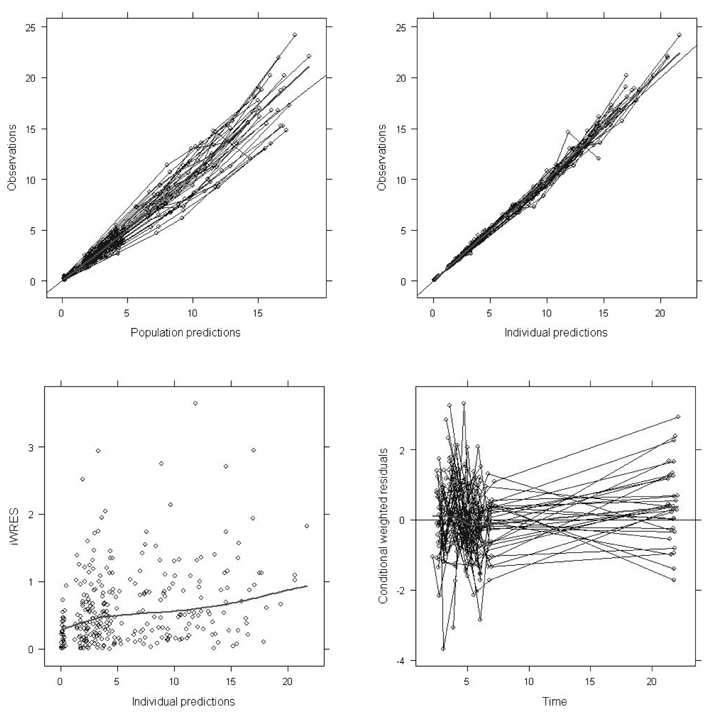
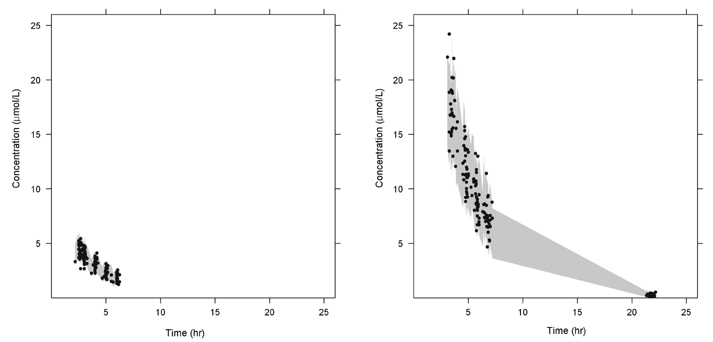
- Busulfan is an antineoplastic agent with a narrow therapeutic window. A post-hoc population pharmacokinetic analysis of a prospective randomized trial for comparison of four-times daily versus once-daily intravenous busulfan was carried out to search for predictive factors of intravenous busulfan (iBu) pharmacokinetics (PK). In this study the population PK of iBu was characterized to provide suitable dosing recommendations. Patients were randomized to receive iBu, either as 0.8 mg/kg every 6 h or 3.2 mg/kg daily over 4 days prior to hematopoietic stem cell transplantation. In total, 295 busulfan concentrations were analyzed with NONMEM. Actual body weight and sex were significant covariates affecting the PK of iBu. Sixty patients were included in the study (all Korean; 23 women, 37 men; mean [SD] age, 36.5 [10.9] years; weight, 66.5 [11.3] kg). Population estimates for a typical patient weighing 65 kg were: clearance (CL) 7.6 l/h and volume of distribution (Vd) 32.2 l for men and 29.1 L for women. Inter-individual random variabilities of CL and Vd were 16% and 9%. Based on a CL estimate from the final PK model, a simple dosage scheme to achieve the target AUC0-inf (defined as median AUC0-inf with a once-daily dosage) of 26.18 mg/lxhr, was proposed: 24.79xABW0.5 mg q24h, where ABW represents the actual body weight in kilograms. The dosing scheme reduced the unexplained interindividual variabilities of CL and Vd of iBu with ABW being a significant covariate affecting clearance of iBU. We propose a new simple dosing scheme for iBu based only on ABW.
MeSH Terms
Figure
Cited by 1 articles
-
Prospective validation of a novel dosing scheme for intravenous busulfan in adult patients undergoing hematopoietic stem cell transplantation
Sang-Heon Cho, Jung-Hee Lee, Hyeong-Seok Lim, Kyoo-Hyung Lee, Dae-Young Kim, Sangmin Choe, Kyun-Seop Bae, Je-Hwan Lee
Korean J Physiol Pharmacol. 2016;20(3):245-251. doi: 10.4196/kjpp.2016.20.3.245.
Reference
-
1. Schuler US, Ehrsam M, Schneider A, Schmidt H, Deeg J, Ehninger G. Pharmacokinetics of intravenous busulfan and evaluation of the bioavailability of the oral formulation in conditioning for haematopoietic stem cell transplantation. Bone Marrow Transplant. 1998. 22:241–244.2. Ehrsson H, Hassan M, Ehrnebo M, Beran M. Busulfan kinetics. Clin Pharmacol Ther. 1983. 34:86–89.3. Hassan M, Oberg G, Ehrsson H, Ehrnebo M, Wallin I, Smedmyr B, Tötterman T, Eksborg S, Simonsson B. Pharmacokinetic and metabolic studies of high-dose busulphan in adults. Eur J Clin Pharmacol. 1989. 36:525–530.4. Slattery JT, Clift RA, Buckner CD, Radich J, Storer B, Bensinger WI, Soll E, Anasetti C, Bowden R, Bryant E, Chauncey T, Deeg HJ, Doney KC, Flowers M, Gooley T, Hansen JA, Martin PJ, Mcdonald GB, Nash R, Petersdorf EW, Sanders JE, Schoch G, Stewart P, Storb R, Sullivan KM, Thomas ED, Witherspoon RP, Appelbaum FR. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997. 89:3055–3060.5. Grochow LB. Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens. Semin Oncol. 1993. 20:4 Suppl 4. 18–25.6. Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE, York RC, Lin LS, Devine SM, Geller RB, Heffner LT, Hillyer CD, Holland HK, Winton EF, Saral R. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant. 1996. 17:225–230.7. Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT, Anderlini P, de Lima M, Gajewski J, Champlin RE. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002. 8:477–485.8. Drugs@FDA. cited 2009 11 Sep. Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Overview&DrugName=BUSULFEX.9. Hassan M, Oberg G, Ehrsson H, Ehrnebo M, Wallin I, Smedmyr B, Tötterman T, Eksborg S, Simonsson B. Pharmacokinetic and metabolic studies of high-dose busulphan in adults. Eur J Clin Pharmacol. 1989. 36:525–530.10. Hassan M, Oberg G, Bekassy AN, Aschan J, Ehrsson H, Ljungman P, Lönnerholm G, Smedmyr B, Taube A, Wallin I, et al. Pharmacokinetics of high-dose busulphan in relation to age and chronopharmacology. Cancer Chemother Pharmacol. 1991. 28:130–134.11. Grochow LB, Jones RJ, Brundrett RB, Braine HG, Chen TL, Saral R, Santos GW, Colvin OM. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol. 1989. 25:55–61.12. Ryu SG, Lee JH, Choi SJ, Lee JH, Lee YS, Seol M, Hur EH, Lee SH, Bae KS, Noh GJ, Lee MS, Yun SC, Han SB, Lee KH. Randomized comparison of four-times-daily versus once-daily intravenous busulfan in conditioning therapy for hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007. 13:1095–1105.13. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984. 2:187–193.14. Gibbs JP, Gooley T, Corneau B, Murray G, Stewart P, Appelbaum FR, Slattery JT. The impact of obesity and disease on busulfan oral clearance in adults. Blood. 1999. 93:4436–4440.15. Devine D. Case study number 25 gentamicin therapy. Drug Intell Clin Pharm. 1974. 8:650–655.16. Vaughan WP, Carey D, Perry S, Westfall AO, Salzman DE. A limited sampling strategy for pharmacokinetic directed therapy with intravenous busulfan. Biol Blood Marrow Transplant. 2002. 8:619–624.17. Arand M, Mühlbauer R, Hengstler J, Jäger E, Fuchs J, Winkler L, Oesch F. A multiplex polymerase chain reaction protocol for the simultaneous analysis of the glutathione S-transferase GSTM1 and GSTT1 polymorphisms. Anal Biochem. 1996. 236:184–186.18. dos Reis EO, Vianna-Jorge R, Suarez-Kurtz G, Lima EL, Azevedo Dde A. Development of a rapid and specific assay for detection of busulfan in human plasma by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2005. 19:1666–1674.19. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976. 16:31–41.20. Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987. 317:1098.21. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989. 5:303–311.22. Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978. 93:62–66.23. Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Rep. 1970. 54:225–235.24. Boyd E. The Growth of the Surface Area of the Human Body. 1935. London: University of Minnesota.25. Mandema JW, Verotta D, Sheiner LB. Building population pharmacokinetic--pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm. 1992. 20:511–528.26. Jonsson EN, Karlsson MO. Xpose--an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999. 58:51–64.27. Wählby U, Jonsson EN, Karlsson MO. Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn. 2001. 28:231–252.28. Cook RD, Weisberg S. Residuals and Influence in Regression. 1982. New York: Chapman and Hall.29. Christensen R, Pearson LM, Johnson W. Case-Deletion diagnostics for mixed models. Technometrics. 1992. 34:38–45.30. Nguyen L, Leger F, Lennon S, Puozzo C. Intravenous busulfan in adults prior to haematopoietic stem cell transplantation: a population pharmacokinetic study. Cancer Chemother Pharmacol. 2006. 57:191–198.31. Booth BP, Rahman A, Dagher R, Griebel D, Lennon S, Fuller D, Sahajwalla C, Mehta M, Gobburu JV. Population pharmacokinetic-based dosing of intravenous busulfan in pediatric patients. J Clin Pharmacol. 2007. 47:101–111.32. Takama H, Tanaka H, Nakashima D, Ueda R, Takaue Y. Population pharmacokinetics of intravenous busulfan in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006. 37:345–351.33. Sandström M, Karlsson MO, Ljungman P, Hassan Z, Jonsson EN, Nilsson C, Ringden O, Oberg G, Bekassy A, Hassan M. Population pharmacokinetic analysis resulting in a tool for dose individualization of busulphan in bone marrow transplantation recipients. Bone Marrow Transplant. 2001. 28:657–664.34. Schiltmeyer B, Klingebiel T, Schwab M, Mürdter TE, Ritter CA, Jenke A, Ehninger G, Gruhn B, Würthwein G, Boos J, Hempel G. Population pharmacokinetics of oral busulfan in children. Cancer Chemother Pharmacol. 2003. 52:209–216.35. Poonkuzhali B, Chandy M, Srivastava A, Dennison D, Krishnamoorthy R. Glutathione S-transferase activity influences busulfan pharmacokinetics in patients with beta thalassemia major undergoing bone marrow transplantation. Drug Metabolism and Disposition. 2001. 29:264–267.36. Gibbs JP, Liacouras CA, Baldassano RN, Slattery JT. Up-regulation of glutathione S-transferase activity in enterocytes of young children. Drug Metab Dispos. 1999. 27:1466–1469.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prospective validation of a novel dosing scheme for intravenous busulfan in adult patients undergoing hematopoietic stem cell transplantation
- PKconverter: R package to convert the pharmacokinetic parameters
- Physiological spaces and multicompartmental pharmacokinetic models
- Pharmacodynamic principles and target concentration intervention
- Busulfan lung: report of 2 cases