Transl Clin Pharmacol.
2015 Dec;23(2):38-41. 10.12793/tcp.2015.23.2.38.
Physiological spaces and multicompartmental pharmacokinetic models
- Affiliations
-
- 1Department of Pharmacology, Feinberg School of Medicine, Northwestern University Chicago, Illinois, USA. art_atkinson@msn.com
- KMID: 2351805
- DOI: http://doi.org/10.12793/tcp.2015.23.2.38
Abstract
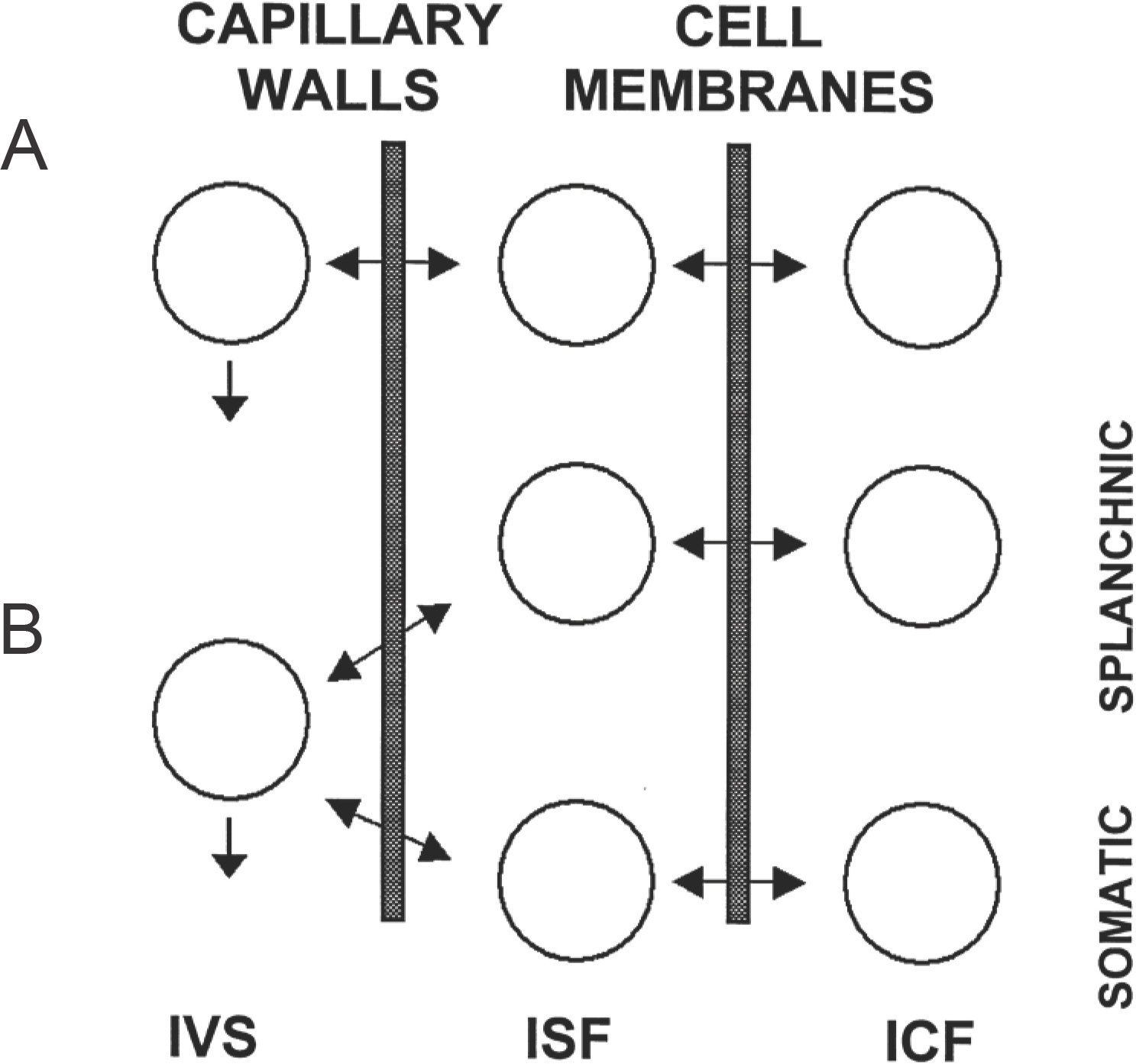
- The idea of body compartments has its origins in physiology and antedates their use in both physiologically-based predictive pharmacokinetic models and in the simpler compartmental models used to analyze pharmacokinetic data. Whereas physiologically-based pharmacokinetics has evolved to use increasingly sophisticated organ-based models, most compartmental models for data analysis are used without regard for their underlying physiological basis. However, detailed analysis of inulin and urea kinetics has offered some understanding of the physiological basis underlying some three-compartment pharmacokinetic models. In addition, these simple models have yielded new insight into physiological phenomena.
MeSH Terms
Figure
Cited by 1 articles
-
“Physiological spaces and multicompartmental pharmacokinetic models”: Fundamentals that pharmacokinetics textbooks do not tell you
Dong-Seok Yim
Transl Clin Pharmacol. 2015;23(2):35-37. doi: 10.12793/tcp.2015.23.2.35.
Reference
-
References
1. Bernard C. Point de vue du milieu extérieur et du milieu intéreur, Chapter 19, 1º in: Bernard C. Principes de médecine expérimentale: ou d l' expéi-mentation appliqué à la pathologie et à la thérapeutique, written between 1859–1877, re-published by Les Presses Universitaires de France. 1947. 279.2. Novak LP. Total body water in man. in: Bergner P-EE, Lushbaugh CC (eds) Compartments pools and spaces in medical physiology. US Atomic Energy Commission, Oak Ridge. 1967. 197–216.3. Fairbanks VF, Tauxe WN. Plasma and erythrocyte volumes in obesity, polycythemia, and related conditions. in: Bergner P-EE, Lushbaugh CC (eds) Compartments pools and spaces in medical physiology. US Atomic Energy Commission, Oak Ridge. 1967. 283–298.4. Keith NM, Rowntree LG, Geraghty JT. A method for the determination of plasma and blood volume. Arch Intern Med. 1915; 15:543–578.
Article5. Painter EE. Total body water in the dog. Am J Physiol. 1940; 129:744–754.
Article6. Schloerb PR, Friis-Hansen BJ, Edelman IS, Solomon AK, Moore FD. The measurement of total body water in the human subject by deuterium oxide dilution with a consideration of the dynamics of deuterium distribution. J Clin Invest. 1950; 29:1296–1310.7. Kornberg HL, Davies RE. Measurement of total body water with urea. Nature. 1952; 169:502–503.8. Gaudino M. Kinetics of distribution of inulin between two body water compartments. Proc Soc Exper Biol Med. 1949; 4:672–674.
Article9. Paalzow LK. Torsten Teorell, the father of pharmacokinetics. Upsala J Med Sci. 1995; 100:41–46.
Article10. Teorell T. Kinetics of distribution of substances administered to the body. 1. The extravascular modes of administration. Arch Int Pharmacodyn Ther. 1937; 57:225–240.11. Atkinson AJ Jr. Individualization of drug therapy: an historical perspective. Transl Clin Pharmacol. 2014; 22:52–54.
Article12. Berlin NI, Berman M, Berk PD, Phang JM, Waldmann TA. The application of multicompartmental analysis to problems of clinical medicine. Ann Intern Med. 1968; 68:423–448.
Article13. Oppenheimer JH, Schwartz HL, Surks MI. Determination of common parameters of iodothyronine metabolism and distribution in man by noncompartmental methods. J Clin Endocrin Metab. 1975; 41:319–324.14. Price HL, Kovnat PJ, Safer JN, Conner EH, Price ML. The uptake of thiopental by body tissues and its relation to the duration of narcosis. Clin Pharmacol Ther. 1960; 1:16–22.
Article15. Bischoff KB, Dedrick RL. Thiopental pharmacokinetics. J Pharm Sci. 1968. 1341–1351.
Article16. Benowitz N, Forsyth RP, Melmon KL, Rowland M. Lidocaine disposition kinetics in monkey and man 1. Prediction by a perfusion model. Clin Pharmacol Ther. 1977; 16:87–109.17. Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011; 51:45–71.
Article18. Zhao P, Zhang L, Grillo JA, Kim Q, Bullock JM, Moon YJ, et al. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther. 2011; 89:259–267.
Article19. Henthorn TK, Avram MJ, Frederiksen MC, Atkinson AJ Jr. Heterogeneity of interstitial fluid space demonstrated by simultaneous kinetic analysis of the distribution and elimination of inulin and gallamine. J Pharmacol Exp Ther. 1982; 222:389–394.20. Sherwin RS, Kramer KJ, Tobin JD, Insel PA, Liljenquist JE, Berman M, et al. A model of the kinetics of insulin in man. J Clin Invest. 1974; 53:1481–1492.
Article21. Bowsher DJ, Avram MJ, Frederiksen MC, Asada A, Atkinson AJ Jr. Urea distribution kinetics analyzed by the simultaneous injection of urea and inulin: Demonstration that transcapillary exchange is rate limiting. J Pharmacol Exp Ther. 1984; 230:269–274.22. Sedek GS, Ruo TI, Frederiksen MC, Frederiksen JW, Shin S-R, Atkinson AJ Jr. Splanchnic tissues are a major part of the rapid distribution spaces of inulin, urea and theophylline. J Pharmacol Ther. 1989; 25:1026–1031.23. Renkin EM. Effects of blood flow on diffusion kinetics in isolated perfused hindlegs of cats: a double circulation hypothesis. Am J Physiol. 1955; 183:125–136.24. Odeh YK, Wang Z, Ruo TI, Wang T, Frederiksen MC, Pospisil PA, et al. Simultaneous analysis of inulin and 15N2-urea kinetics in humans. Clin Pharmacol Ther. 1993; 53:419–425.
Article25. Larsen PR, Atkinson AJ Jr, Wellman HN, Goldsmith RE. The effect of diphenylhydantoin on thyroxine metabolism in man. J Clin Invest. 1970; 49:1266–1279.26. Belknap SM, Nelson JE, Ruo TI, Frederiksen MC, Worwag EM, Shin S-G, et al. Theophylline distribution kinetics analyzed by reference to simultaneously injected urea and inulin. J Pharmacol Exp Ther. 1987; 243:963–969.27. Camarata SJ, Weil MH, Hanashiro PK, Shubin H. Cardiac arrest in the critically ill. I. A study of predisposing causes in 132 patients. Circulation. 1971; 44:688–695.
Article28. Atkinson AJ Jr.Umans JG. Pharmacokinetic studies in hemodialysis patients. Clin Pharmacol Ther. 2009; 86:548–552.
Article29. Stec GP, Atkinson AJ Jr, Nevin MJ, Thenot JP, Ruo TI, Gibson TP, et al. N-Acetylprocainamide pharmacokinetics in functionally anephric patients before and after perturbation by hemodialysis. Clin Pharmacol Ther. 1979; 26:618–628.
Article30. Bowsher DJ, Krejcie TC, Avram MJ, Chow MJ, del Greco F, Atkinson AJ Jr. Reduction in slow intercompartmental clearance of urea during dialysis. J Lab Clin Med. 1985; 105:489–497.31. Sidhom OA, Odeh YK, Krumlovsky FA, Budris WA, Wang Z, Pospisil PA, et al. Low-dose prazosin in patients with muscle cramps during hemodialysis. Clin Pharmacol Ther. 1994; 56:445–451.
Article32. Henthorn TK, Krejcie TC, Avram MJ. Early drug distribution: A generally neglected aspect of pharmacokinetics of particular relevance to intravenously administered anesthetic agents. Clin Pharmacol Ther. 2008; 84:18–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- "Physiological spaces and multicompartmental pharmacokinetic models": Fundamentals that pharmacokinetics textbooks do not tell you
- PKconverter: R package to convert the pharmacokinetic parameters
- Obesity and anesthetic pharmacology: simulation of target-controlled infusion models of propofol and remifentanil
- Compatibility Study between Physiologically Based Pharmacokinetic (PBPK) and Compartmental PK Model Using Lumping Method: Application to the Voriconazole Case
- Derivation of pharmacokinetic equations