Transl Clin Pharmacol.
2019 Jun;27(2):73-79. 10.12793/tcp.2019.27.2.73.
PKconverter: R package to convert the pharmacokinetic parameters
- Affiliations
-
- 1Department of Statistics, Ewha Womans University, Seoul 03760, Korea. lee.eunk@ewha.ac.kr
- KMID: 2457575
- DOI: http://doi.org/10.12793/tcp.2019.27.2.73
Abstract
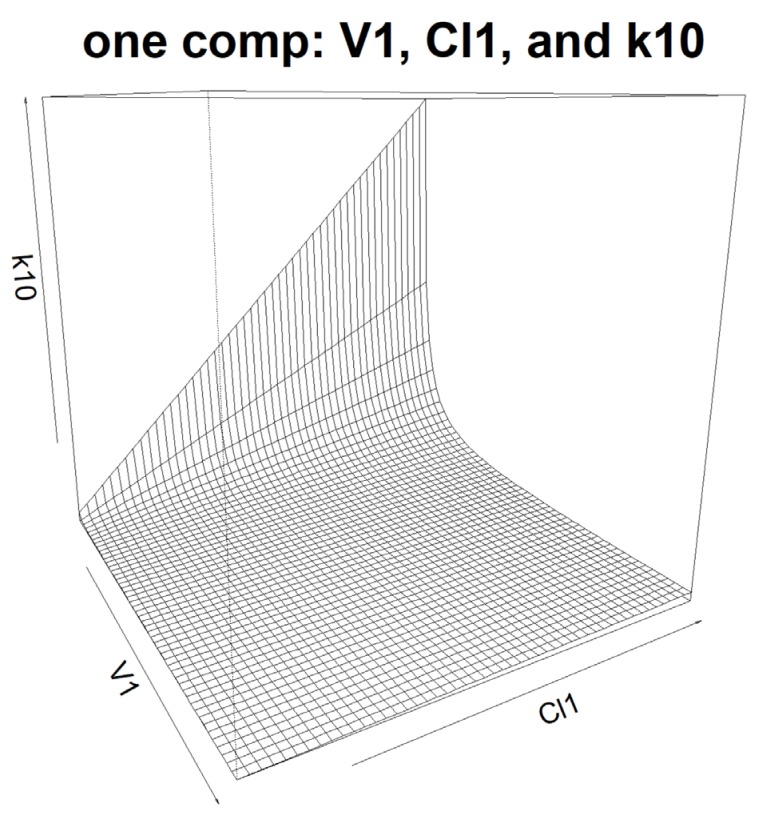
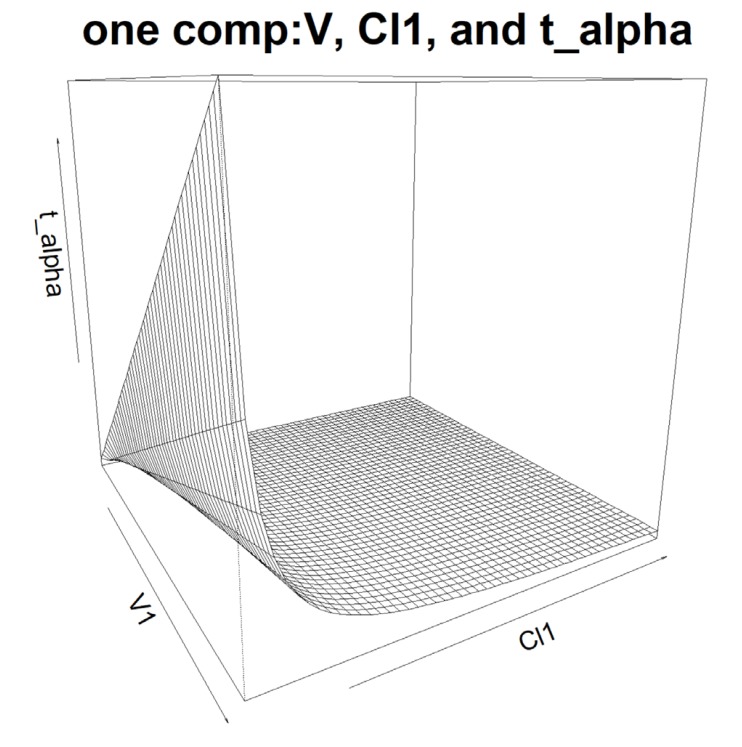
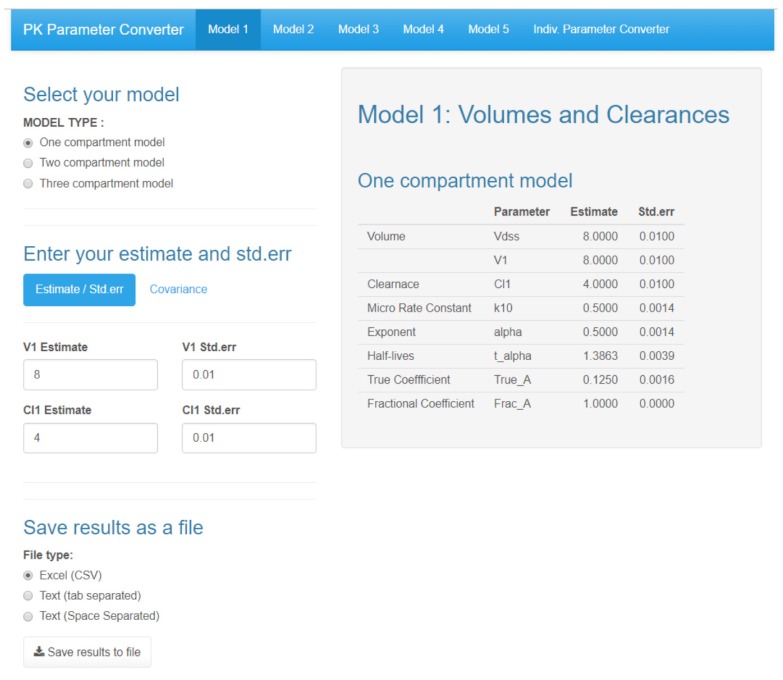
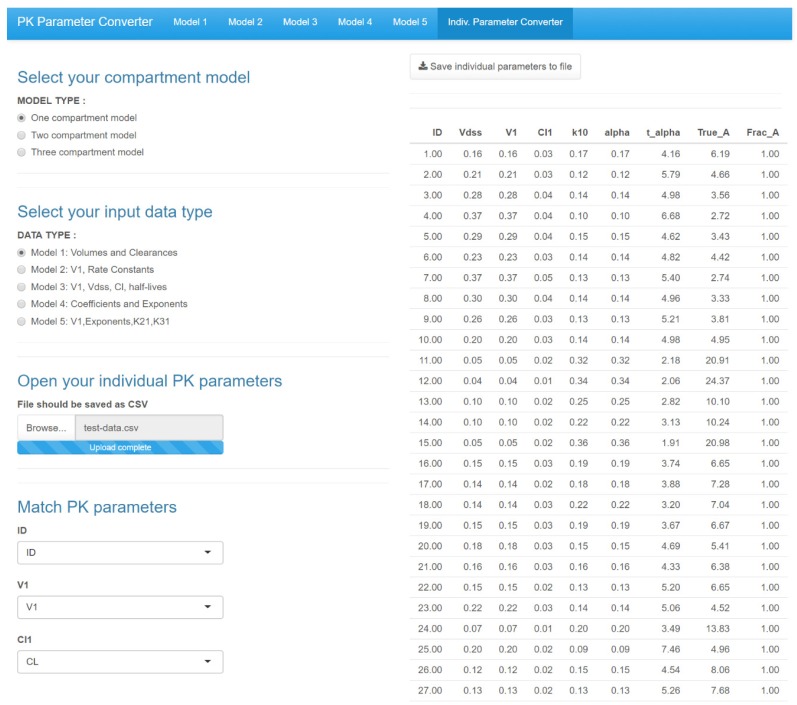
- Population pharmacokinetic analysis and modeling procedures typically require estimates of both population and individual pharmacokinetic parameters. However, only some of these parameters are contained in models and only parameters in the model can be estimated. In this paper, we introduce a new R package, PKconverter, to calculate pharmacokinetic parameters using the relationships among them. After fitting the model, other parameters can be calculated from the functional relationship among the parameters. PKconverter provides the functions to calculate whole parameters along with a Shiny application for converting the parameters. With this package, it is also possible to calculate the standard errors of the other parameters that are not in the model and estimate individual parameters simultaneously.
Figure
Reference
-
1. Chang W, Cheng J, Allaire JJ, Xie Y, McPherson J. Shiny: web application framework for R. R package version 0.11. 2015.2. DiPiro JT. Concepts in clinical pharmacokinetics. ASHP;2010.3. Rowland M, Tozer TN, Derendorf H, Hochhaus G. Clinical pharmacokinetics and pharmacodynamics: concepts and applications. Philadelphia, PA: Wolters Kluwer Health/Lippincott William & Wilkins;2011.4. Gabrielsson J, Weiner D. Pharmacokinetic and pharmacodynamic data analysis: concepts and applications, volume 2. CRC Press;2001.5. Benet LZ, Zia-Amirhosseini P. Basic principles of pharmacokinetics. Toxicol Pathol. 1995; 23:115–123. DOI: 10.1177/019262339502300203. PMID: 7569664.
Article6. Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development. CPT Pharmacometrics Syst Pharmacol. 2012; 1:1–14. DOI: 10.1038/psp.2012.4.
Article7. Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development—part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol. 2013; 2:1–14. DOI: 10.1038/psp.2013.14.
Article8. Shargel L, Andrew B, Wu-Pong S. Applied biopharmaceutics & pharmacokinetics. Appleton & Lange Stamford;1999.9. Lehmann EL. Elements of large-sample theory. Springer Science & Business Media;2004.10. Taboga T. Lectures on probability theory and mathematical statistics. CreateSpace Independent Publishing Platform;2012.11. Casella G, Berger RL. Statistical inference, volume 2. Pacific Grove, CA: Duxbury;2002.12. Cho H. PKconverter: shiny application to convert the PK parameters. Ewha Womans University;2018.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Caffsim: simulation of plasma caffeine concentrations implemented as an R package and Web-applications
- Development of Physiologically Based Pharmacokinetic Model for Several Volatile Organic Compounds
- A semi-compartmental model describing the pharmacokinetic-pharmacodynamic relationship
- Contributing Factors on Pharmacokinetic Variability in Critically Ill Neonates
- Analytical solution of linear multi-compartment models with non-zero initial condition and its implementation with R