Anat Cell Biol.
2012 Jun;45(2):86-91. 10.5115/acb.2012.45.2.86.
Vasculosyncytial membrane in relation to syncytial knots complicates the placenta in preeclampsia: a histomorphometrical study
- Affiliations
-
- 1Department of Anatomy, Narayana Medical College, Nellore, India. lesanshar@gmail.com
- 2Department of Anatomy, MNR Medical College, Sangareddy, India.
- 3Department of Pathology, Narayana Medical College, Nellore, Andhra Pradesh, India.
- KMID: 2005870
- DOI: http://doi.org/10.5115/acb.2012.45.2.86
Abstract
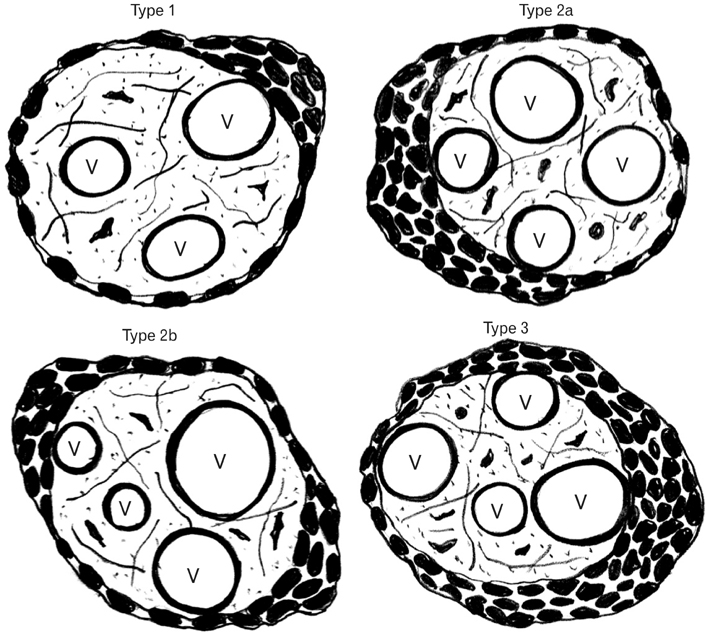
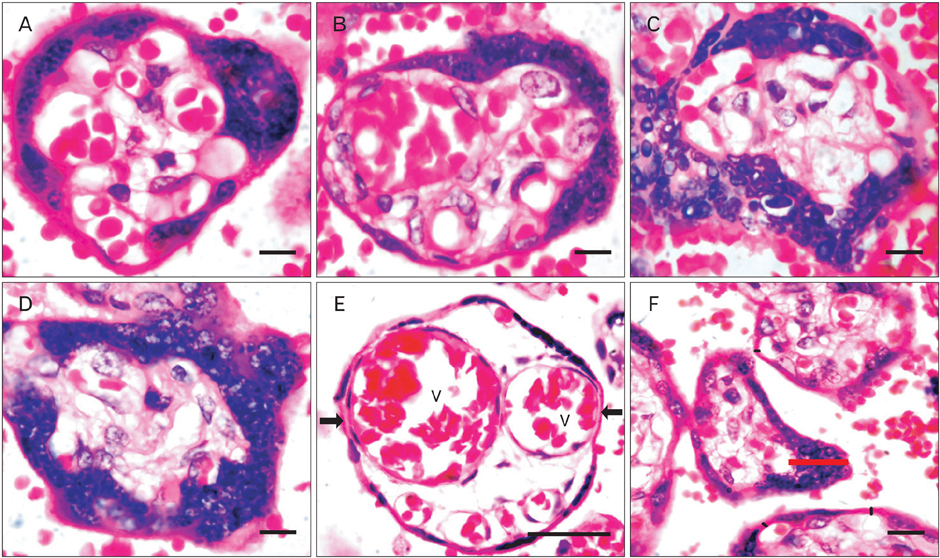
- The vasculosyncytial membrane (VSM), primary site of fetomaternal exchange is formed when syncytiotrophoblast surrounds the terminal villi and make a close contact with capillaries. Some syncytiotrophoblast forms thin single layer of villous and some syncytial nuclei become piled up to form the syncytial knots (SKs). Undoubtedly there is a clear-cut inverse relation between villous VSM and fetal hypoxia. In preeclampsia (PE) the hypoxia injury disrupts the syncytial architecture which in turn initiates other complications of PE. Present study was designed to observe the morphological and histomorphometric features of 84 placentas from control and PE (42 each) collected from Department of Obstetrics and Gynecology. Neonatal weight and placental weight were reduced in PE than the controls but the feto-placental index did not differ. The SK density and VSM thickness was found to be increased and was statistically significant in PE cases. In relation to SKs, the VSM thickness was twofold increased than the controls and was statistically significant. The SKs in the present study were classified as type-1, 2a, 2b, and 3. Type 1 was found to be 62% in control and 47% in PE, type 2a and 2b were 38% in control and 37% in PE, and type 3 was in 8% of PE cases. All the parameters of present study reveal the adverse effects of PE influencing on both morphological and microscopical features of the placenta resulting in fetal hypoxia.
MeSH Terms
Figure
Reference
-
1. Brown MA, Hague WM, Higgins J, Lowe S, McCowan L, Oats J, Peek MJ, Rowan JA, Walters BN. Austalasian Society of the Study of Hypertension in Pregnancy. The detection, investigation and management of hypertension in pregnancy: full consensus statement. Aust N Z J Obstet Gynaecol. 2000. 40:139–155.2. Milne F, Redman C, Walker J, Baker P, Bradley J, Cooper C, de Swiet M, Fletcher G, Jokinen M, Murphy D, Nelson-Piercy C, Osgood V, Robson S, Shennan A, Tuffnell A, Twaddle S, Waugh J. The pre-eclampsia community guideline (PRECOG): how to screen for and detect onset of pre-eclampsia in the community. BMJ. 2005. 330:576–580.3. Walker JJ. Pre-eclampsia. Lancet. 2000. 356:1260–1265.4. Roberts JM, Cooper DW. Pathogenesis and genetics of preeclampsia. Lancet. 2001. 357:53–56.5. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005. 365:785–799.6. Xiong X, Buekens P, Pridjian G, Fraser WD. Pregnancy-induced hypertension and perinatal mortality. J Reprod Med. 2007. 52:402–406.7. Podjarny E, Losonczy G, Baylis C. Animal models of preeclampsia. Semin Nephrol. 2004. 24:596–606.8. Heazell A, Harris L, Forbes K, Crocker I. Placental cell turnover in health and disease. Rev Gynaecol Perinat Pract. 2006. 6:80–86.9. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004. 350:672–683.10. Levine RJ, Karumanchi SA. Circulating angiogenic factors in preeclampsia. Clin Obstet Gynecol. 2005. 48:372–386.11. Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD. Morphometric placental villous and vascular abnormalities in earlyand late-onset pre-eclampsia with and without fetal growth restriction. BJOG. 2006. 113:580–589.12. Kliman HJ, Segel L. The placenta may predict the baby. J Theor Biol. 2003. 225:143–145.13. Benirschke K, Kaufmann P. Pathology of the human placenta. 2000. New York: Springer-Verlag;55–56.14. Desforges M, Sibley CP. Placental nutrient supply and fetal growth. Int J Dev Biol. 2010. 54:377–390.15. Baergen RN. Manual of Benirschke and Kaufmann's pathology of the human placenta. 2005. 2nd ed. New York: Springer Science & Business Media;72–74.16. Jain K, Kavi V, Raghuveer CV, Sinha R. Placental pathology in pregnancy-induced hypertension (PIH) with or without intrauterine growth retardation. Indian J Pathol Microbiol. 2007. 50:533–537.17. Spanos S, Rice S, Karagiannis P, Taylor D, Becker DL, Winston RM, Hardy K. Caspase activity and expression of cell death genes during development of human preimplantation embryos. Reproduction. 2002. 124:353–363.18. Huppertz B. Placental villous trophoblast: the altered balance between proliferation and apoptosis triggers pre-eclampsia. J Reproduktionsmed Endokrinol. 2006. 3:103–108.19. Aplin JD. Developmental cell biology of human villous trophoblast: current research problems. Int J Dev Biol. 2010. 54:323–329.20. Mihu CM, Suşman S, Rus Ciucă D, Mihu D, Costin N. Aspects of placental morphogenesis and angiogenesis. Rom J Morphol Embryol. 2009. 50:549–557.21. Kierszenbaum AL. Histology and cell biology: an introduction to pathology. 2007. 2nd ed. Philadelphia: Mosby-Elsevier;635–661.22. Burton GJ, Charnock-Jones DS, Jauniaux E. Regulation of vascular growth and function in the human placenta. Reproduction. 2009. 138:895–902.23. Fox H, Sebire NJ. Pathology of the placenta. 2007. 3rd ed. Philadalphia, London: W. B. Saunders;155.24. Huppertz B, Kadyrov M, Kingdom JC. Apoptosis and its role in the trophoblast. Am J Obstet Gynecol. 2006. 195:29–39.25. Abumaree MH, Stone PR, Chamley LW. An in vitro model of human placental trophoblast deportation/shedding. Mol Hum Reprod. 2006. 12:687–694.26. Pardi G, Marconi AM, Cetin I. Pathophysiology of intrauterine growth retardation: role of the placenta. Acta Paediatr Suppl. 1997. 423:170–172.27. Huppertz B. The anatomy of the normal placenta. J Clin Pathol. 2008. 61:1296–1302.28. Gill JS, Salafia CM, Grebenkov D, Vvedensky DD. Modeling oxygen transport in human placental terminal villi. J Theor Biol. 2011. 291:33–41.29. Ferrazzani S, Merola A, De Carolis S, Carducci B, Paradisi G, Caruso A. Birth weight in pre-eclamptic and normotensive twin pregnancies: an analysis of discordance and growth restriction. Hum Reprod. 2000. 15:210–217.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression and Localization of the PlGF mRNA in Preeclamptic Placentas
- Nitrous oxide synthase expression in placenta of preeclampsia
- Expression of 14-3-3 proteins and Bax in the placenta of preeclampsia
- Umbilical artery blood gas analysis and its relationship with the placenta to birth weight ratios at birth in preeclampsia and small for gestational age
- Differential expression of Matrix Metalloproteinase (MMP)-2, -9 in normal and severe preeclamptic human placentas