Korean J Urol.
2014 May;55(5):335-340. 10.4111/kju.2014.55.5.335.
Efficacy and Safety of the Selective alpha1A-Adrenoceptor Blocker Silodosin for Severe Lower Urinary Tract Symptoms Associated With Benign Prostatic Hyperplasia: A Prospective, Single-Open-Label, Multicenter Study in Korea
- Affiliations
-
- 1Department of Urology, Yeungnam University Hospital, Daegu, Korea.
- 2Department of Urology, Hallym University Medical Center, Seoul, Korea.
- 3Department of Urology, Pusan National University Hospital, Busan, Korea.
- 4Department of Urology, Seoul National University Hospital, Seoul, Korea.
- 5Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 6Department of Urology, Seoul St. Mary's Hospital, Seoul, Korea.
- 7Department of Urology, Korea University Guro Hospital, Seoul, Korea.
- 8Department of Urology, Chonbuk National University Medical School, Jeonju, Korea.
- 9Institute for Medical Sciences, Chonbuk National University, Jeonju, Korea.
- 10Biomedical Research Institute and Clinical Trial Center of Medical Device of Chonbuk National University Hospital, Jeonju, Korea.
- 11Department of Urology, Asan Medical Center, Seoul, Korea.
- 12Department of Urology, Chonnam National University Medical School, Gwangju, Korea. kpark@chonnam.ac.kr
- KMID: 1988411
- DOI: http://doi.org/10.4111/kju.2014.55.5.335
Abstract
- PURPOSE
To evaluate the efficacy and safety of silodosin 8 mg once daily in a 12-week treatment of subjects with severe lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH).
MATERIALS AND METHODS
A total of 100 subjects from 10 urology centers in Korea were included in this study. The inclusion criteria were as follows: age > or =50 years, International Prostate Symptom Score (IPSS) > or =20, quality of life (QoL) score > or =3, urine volume > or =120 mL and maximal urinary flow rate (Qmax) <15 mL/s, and postvoid residual volume (PVR) <100 mL. We assessed the improvement of LUTS with change in IPSS, QoL score, Qmax, PVR, and adverse events at baseline and 4 and 12 weeks after treatment with silodosin 8 mg once daily.
RESULTS
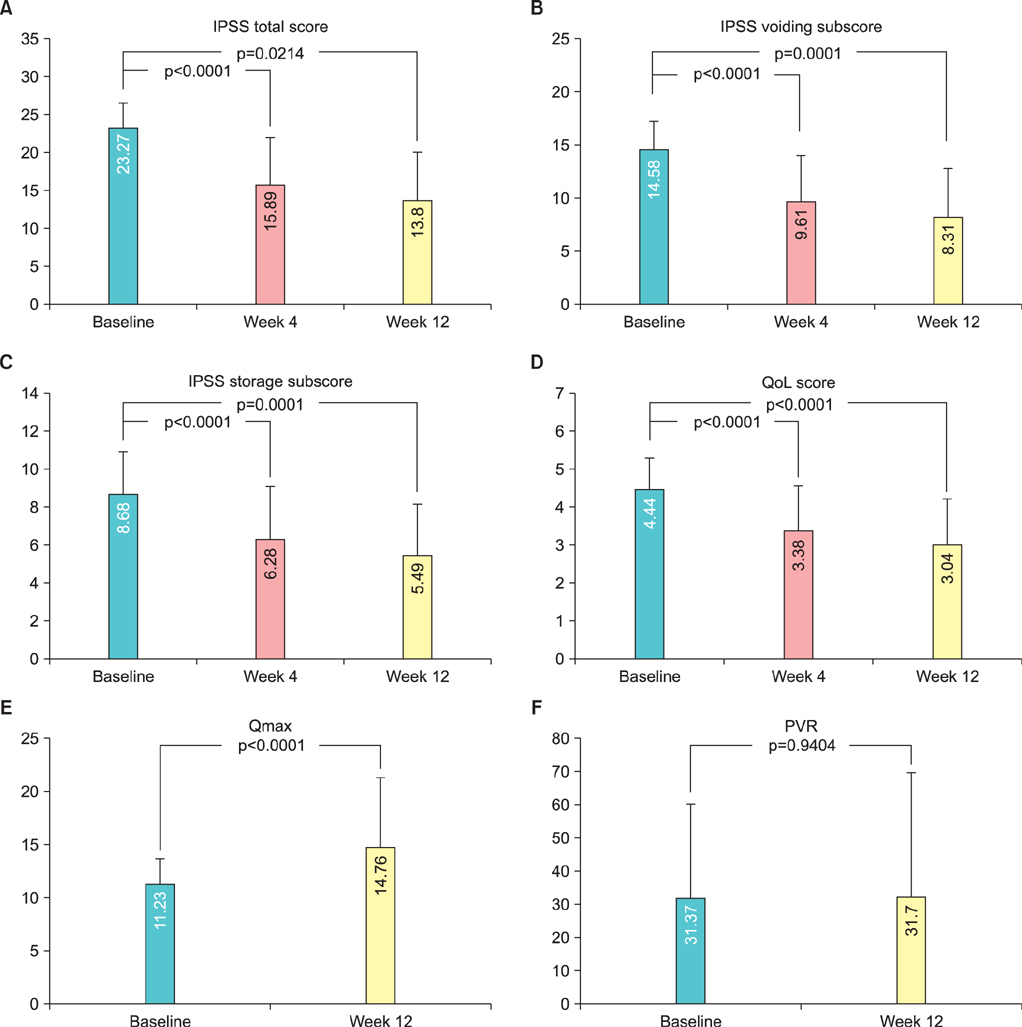
The IPSS values were 23.27+/-3.34, 15.89+/-6.26, and 13.80+/-6.31 at baseline, 4, and 12 weeks, respectively, with significant improvements (p<0.0001, p=0.0214, respectively). QoL scores were 4.44+/-0.85, 3.38+/-1.20, and 3.04+/-1.20 at baseline, 4, and 12 weeks, respectively, and the differences were statistically significant (p<0.0001). There was a significant difference in Qmax between baseline and 12 weeks (p<0.0001) but not in PVR (p=0.9404) during the clinical trial. The most frequent adverse event in this study was ejaculation failure with 13 cases. However, no subject dropped out because of ejaculation failure, and in 12 of the 13 cases it was fully resolved without further treatment.
CONCLUSIONS
Silodosin 8 mg once daily may be effective and safe in Korean patients with severe LUTS associated with BPH.
Keyword
MeSH Terms
Figure
Reference
-
1. Wasserman NF. Benign prostatic hyperplasia: a review and ultrasound classification. Radiol Clin North Am. 2006; 44:689–710.2. Thorpe A, Neal D. Benign prostatic hyperplasia. Lancet. 2003; 361:1359–1367.3. Madersbacher S, Alivizatos G, Nordling J, Sanz CR, Emberton M, de la Rosette JJ. EAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines). Eur Urol. 2004; 46:547–554.4. Fine SR, Ginsberg P. Alpha-adrenergic receptor antagonists in older patients with benign prostatic hyperplasia: issues and potential complications. J Am Osteopath Assoc. 2008; 108:333–337.5. Lepor H. Alpha blockers for the treatment of benign prostatic hyperplasia. Rev Urol. 2007; 9:181–190.6. Tatemichi S, Kobayashi K, Maezawa A, Kobayashi M, Yamazaki Y, Shibata N. Alpha1-adrenoceptor subtype selectivity and organ specificity of silodosin (KMD-3213). Yakugaku Zasshi. 2006; 126(Spec no.):209–216.7. Schilit S, Benzeroual KE. Silodosin: a selective alpha1A-adrenergic receptor antagonist for the treatment of benign prostatic hyperplasia. Clin Ther. 2009; 31:2489–2502.8. Chapple CR, Montorsi F, Tammela TL, Wirth M, Koldewijn E, Fernandez Fernandez E, et al. Silodosin therapy for lower urinary tract symptoms in men with suspected benign prostatic hyperplasia: results of an international, randomized, double-blind, placebo- and active-controlled clinical trial performed in Europe. Eur Urol. 2011; 59:342–352.9. Yoshida M, Kudoh J, Homma Y, Kawabe K. Safety and efficacy of silodosin for the treatment of benign prostatic hyperplasia. Clin Interv Aging. 2011; 6:161–172.10. Kawabe K, Yoshida M, Homma Y. Silodosin Clinical Study Group. Silodosin, a new alpha1A-adrenoceptor-selective antagonist for treating benign prostatic hyperplasia: results of a phase III randomized, placebo-controlled, double-blind study in Japanese men. BJU Int. 2006; 98:1019–1024.11. Marks LS, Gittelman MC, Hill LA, Volinn W, Hoel G. Rapid efficacy of the highly selective alpha1A-adrenoceptor antagonist silodosin in men with signs and symptoms of benign prostatic hyperplasia: pooled results of 2 phase 3 studies. J Urol. 2009; 181:2634–2640.12. Malloy BJ, Price DT, Price RR, Bienstock AM, Dole MK, Funk BL, et al. Alpha1-adrenergic receptor subtypes in human detrusor. J Urol. 1998; 160(3 Pt 1):937–943.13. Rossi M, Roumeguère T. Silodosin in the treatment of benign prostatic hyperplasia. Drug Des Devel Ther. 2010; 4:291–297.14. Capitanio U, Salonia A, Briganti A, Montorsi F. Silodosin in the management of lower urinary tract symptoms as a result of benign prostatic hyperplasia: who are the best candidates. Int J Clin Pract. 2013; 67:544–551.15. Ding H, Du W, Hou ZZ, Wang HZ, Wang ZP. Silodosin is effective for treatment of LUTS in men with BPH: a systematic review. Asian J Androl. 2013; 15:121–128.16. Marks LS, Gittelman MC, Hill LA, Volinn W, Hoel G. Silodosin in the treatment of the signs and symptoms of benign prostatic hyperplasia: a 9-month, open-label extension study. Urology. 2009; 74:1318–1322.17. Murata S, Taniguchi T, Takahashi M, Okada K, Akiyama K, Muramatsu I. Tissue selectivity of KMD-3213, an alpha(1)-adrenoreceptor antagonist, in human prostate and vasculature. J Urol. 2000; 164:578–583.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Benign Prostatic Hyperplasia and Alpha Adrenoceptor Blockers
- Effect of Tamsulosin, a Selective alpha1A-adrenoreceptor Antagonist, in Benign Prostatic Hyperplasia
- Efficacy and Safety of Tamsulosin for Treating Lower Urinary Tract Symptoms Associated with Benign Prostatic Hyperplasia: A Multicenter, Randomized, Controlled, Open-Label Non-Inferiority Study
- Current Trend of the Primary Treatment in Lower Urinary Tract Symptom/Benign Prostatic Hyperplasia
- Induction of Apoptosis by alpha1-Adrenoceptor Antagonists in Benign Prostatic Hyperplasia