Korean J Urol.
2012 Mar;53(3):178-183.
Efficacy and Safety of Tamsulosin for Treating Lower Urinary Tract Symptoms Associated with Benign Prostatic Hyperplasia: A Multicenter, Randomized, Controlled, Open-Label Non-Inferiority Study
- Affiliations
-
- 1Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. ksleedr@skku.edu
- 2Department of Urology, The Catholic University of Korea School of Medicine, Seoul, Korea.
- 3Department of Urology, Seoul National University Hospital, Seoul, Korea.
- 4Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
To compare the efficacy and safety of Sulosin D (PACIFICPHARMA, Korea) and Harnal D (ASTELLAS PHARMA KOREA, Korea) in treating patients with lower urinary tract symptoms associated with benign prostatic hyperplasia (BPH).
MATERIALS AND METHODS
This randomized, controlled, open-label, multicenter non-inferiority study was conducted at four sites in Korea. We randomly assigned 123 patients with an International Prostate Symptom Score (IPSS) > or =12 to receive either Sulosin D or Harnal D treatment for 8 weeks. The primary outcome was the mean change in IPSS from baseline to endpoint. Secondary outcomes were the mean change from baseline to endpoint in IPSS quality of life subscores, maximum uroflowmetry (Qmax), and post-voiding residuals (PVR).
RESULTS
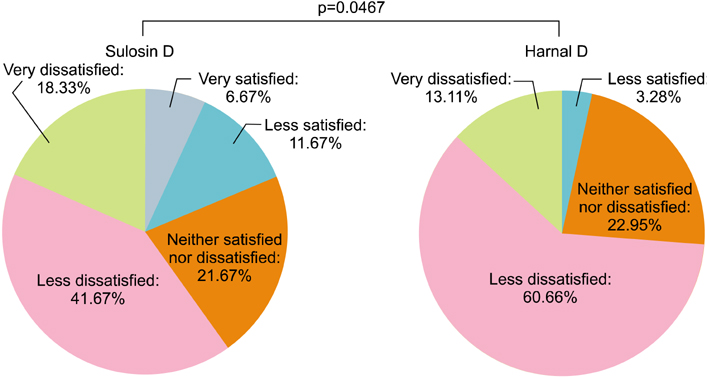
In all, 123 patients were randomly assigned (60 Sulosin D and 63 Harnal D). The changes in the total IPSS from baseline in the Sulosin D- and Harnal D-treated groups were -4.97 and -4.03, respectively. There were significant decreases compared with baseline in both groups. The mean difference (Sulosin D - Harnal D) was -0.91 (with a two-sided 90% confidence interval), inferring that Sulosin D was not inferior to Harnal D. The mean changes in the IPSS subscore, Qmax, and PVR from baseline were comparable between the groups (both p>0.05). During the treatment periods, the incidence of adverse events was 23.33% and 34.92% in the Sulosin D and Harnal D groups, respectively (p=0.1580).
CONCLUSIONS
We demonstrate the non-inferiority of Sulosin D to Harnal D in patients with lower urinary tract symptoms associated with BPH.
Keyword
MeSH Terms
Figure
Reference
-
1. Lepor H. Pathophysiology of lower urinary tract symptoms in the aging male population. Rev Urol. 2005. 7:Suppl 7. S3–S11.2. Homma Y, Kawabe K, Tsukamoto T, Yamanaka H, Okada K, Okajima E, et al. Epidemiologic survey of lower urinary tract symptoms in Asia and Australia using the international prostate symptom score. Int J Urol. 1997. 4:40–46.3. Baazeem A, Elhilali MM. Surgical management of benign prostatic hyperplasia: current evidence. Nat Clin Pract Urol. 2008. 5:540–549.4. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: diagnosis and treatment recommendations. J Urol. 2003. 170(2 Pt 1):530–547.5. Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. Guidelines on the Treatment of Non-neurogenic Male LUTS. 2011. European Association of Urology.6. Barendrecht MM, Koopmans RP, de la Rosette JJ, Michel MC. Treatment of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: the cardiovascular system. BJU Int. 2005. 95:Suppl 4. 19–28.7. Watson V, Ryan M, Brown CT, Barnett G, Ellis BW, Emberton M. Eliciting preferences for drug treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia. J Urol. 2004. 172(6 Pt 1):2321–2325.8. Lee E, Lee C. Clinical comparison of selective and non-selective alpha 1A-adrenoreceptor antagonists in benign prostatic hyperplasia: studies on tamsulosin in a fixed dose and terazosin in increasing doses. Br J Urol. 1997. 80:606–611.9. Lee ES, Lee CW. Effect of tamsulosin, a selective alpha1A-adrenoreceptor antagonist, in benign prostatic hyperplasia. Korean J Urol. 1997. 38:158–166.10. A Randomized, Double-Blind, Placebo-Controlled, Parallel-Design, Piolt Study to Evaluate the Efficacy and Safety of Tadalafil and Tamsulosin Once-a-Day Dosing for 12 weeks in Asian Men With Signs and Symptoms of Benign Prostatic Hyperplasia. Company ELa. From 2007 to Jun 2008. http://www.clinicaltrial.gov/ct2/show/NCT00540124?term=Tadalafil+and+Tamsulosin&rank=1.11. Barry MJ, Williford WO, Change Y, Machi M, Jones KM, Walker-Corkey E, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995. 154:1770–1774.12. Höfner K, Claes H, De Reijke TM, Folkestad B, Speakman . Tamsulosin 0.4 mg once daily: effect on sexual function in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol. 1999. 36:335–341.13. Lee YB. Considering aspects for the revision of current bioequivalence guideline. J Korean Pharmaceut Sci. 2009. 39:233–242. http://img.kisti.re.kr/originalView/originalView.jsp?url=/soc_img/society//kpst/KOJHBZ/2009/v39n4/KOJHBZ_2009_v39n4_233.pdf.14. Chen ML, Shah V, Patnaik R, Adams W, Hussain A, Conner D, et al. Bioavailability and bioequivalence: an FDA regulatory overview. Pharm Res. 2001. 18:1645–1650.15. Shaw SJ, Krauss GL. Generic antiepileptic drugs. Curr Treat Options Neurol. 2008. 10:260–268.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Experience with Combination of Finasteride and Tamsulosin on Benign Prostatic Hyperplasia
- Effect of Tamsulosin, a Selective alpha1A-adrenoreceptor Antagonist, in Benign Prostatic Hyperplasia
- Efficacy and Tolerability of Tamsulosin 0.4 mg in Patients with Symptomatic Benign Prostatic Hyperplasia
- Current Trend of the Primary Treatment in Lower Urinary Tract Symptom/Benign Prostatic Hyperplasia
- Effects of Low-Dose Tamsulosin on Sexual Function in Patients With Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Hyperplasia