J Korean Acad Conserv Dent.
2003 Sep;28(5):385-391. 10.5395/JKACD.2003.28.5.385.
The verification of the MTT assay on the viability of periodontal ligamental cells in rat molars through the histologic examination
- Affiliations
-
- 1Department of Conservative Dentistry, College of dentistry, Yonsei University, Korea. sjlee@yumc.yonsei.ac.kr
- KMID: 1987264
- DOI: http://doi.org/10.5395/JKACD.2003.28.5.385
Abstract
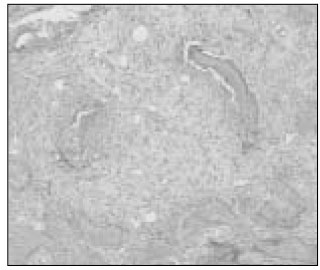
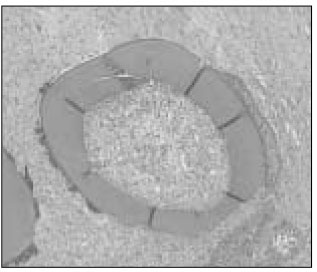
- The purpose of this study is to examine the viability of PDL cells in rat molars by using MTT assay and to verify the MTT assay through the histologic observation. Thirty of Sprague-Dawley white female rats of 4-weeks old with a body weight of about 100 grams were used. Groupings are as follows: Immediate Group : Positive control group(n=10)-after extraction immediately. Dried Group : Negative control group(n=10)-after drying for an hour under warm dry. ViaSpan(R) Group : 1hour ViaSpan(R) group(n=10)-after storing in ViaSpan(R) at 4degrees C for 1hour. Ten teeth of each group were treated as same as above and replanted to the original socket of experimental animals. After two weeks of replantation, all the experimental animals were sacrificed. And after fixation, extracted maxillary jaw was dimineralized. After it was embedded in paraffin, serial section by 5microm was carried out and for construction of specimen, hematoxylin-eosin dye was used. The mean MTT measurement of immediate group(positive control) is 2.81 and the mean measurement of dried group(negative control) is 0.98 which is significant differnt(P<0.05). The mean measurement of ViaSpan(R) group is 2.65 and there is significant difference between dried group and ViaSpan(R) group(P<0.05). However, there is no difference between immediate group and ViaSpan(R) group. The average resorption points of immediate group is 3.03 points. In the dried group, average 6.44 points resorption and 2.68 points showed resorption in the ViaSpan(R) group. Unlike with MTT assay, there was no significant difference between the immediate group and ViaSpan(R) group. The usage of MTT assay as a viable cell marker may give us a better indication of the maintenance of periodontal ligament cell vitality.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Evaluation of periodontal ligament cell viability in rat teeth according to various extra-oral dry storage times using MTT assay
In-Soo Jeon, Eui-Seong Kim, Jin Kim, Seung-Jong Lee
J Korean Acad Conserv Dent. 2006;31(5):398-408. doi: 10.5395/JKACD.2006.31.5.398.
Reference
-
1. Lee SJ, Jung IY, Lee CY, Choi SY, Kum KY. Clinical application of computer-aided rapid prototyping for tooth transplantation. Dent Traumatol. 2001. 17:114–119.
Article2. Andreasen JO, Kristerson L. The effect of limited drying or removal of the periodontal ligament. Periodontal healing after replantation of mature permanent incisors in monkeys. Acta Odontol Scand. 1981. 39:1–13.3. Söder PO, Otteskog P, Andreasen JO, Modeer T. Effect of drying on viability of the periodontal membrane. Scand J Dent Res. 1977. 85:164–168.4. Andreasen JO, Hjorting-Hansen E. Replantation of teeth. I. Radiographic and clinical study of 110 human teeth replanted after accidental loss. Acta Odontol Scand. 1966a. 24:263–286.
Article5. Andreasen JO, Hjorting-Hansen E. Replantation of teeth. II. Histological study of 22 replanted anterior teeth in humans. Acta Odontol Scand. 1966b. 24:287–306.
Article6. Blomlöf L. Milk and saliva as possible storage media for traumatically exarticulated teeth prior to replantation. Swed Dent J Suppl. 1981. 8:1–26.7. Krasner P, Rankow HJ. New philosophy for the treatment of avulsed teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995. 79:616–623.
Article8. Layug ML, Barrett EJ, Kenny DJ. Interim storage of avulsed permanent teeth. J Can Dent Assoc. 1998. 64:357–363. 365–369.9. Harkacz OM Sr, Carnes DL Jr, Walker WA 3rd. Determination of periodontal ligament cell viability in the oral rehydration fluid Gatorade and milks of varying fat content. J Endod. 1997. 23:687–690.
Article10. Ashkenazi M, Sarnat H, Keila S. In vitro viability, mitogenicity and clonogenic capacity of periodontal ligament cells after storage in six different media. Endod Dent Traumatol. 1999. 15:149–156.
Article11. Patel S, Dumsha TC, Sydiskis RJ. Determining periodontal ligament (PDL) cell vitality from exarticulated teeth stored in saline or milk using fluorescein diacetate. Int Endod J. 1994. 27:1–5.
Article12. Hupp JG, Trope M, Mesaros SV, Aukhil I. Tritiated thymidine uptake in periodontal ligament cells of dogs' teeth stored in various media for extended time periods. Endod Dent Traumatol. 1997. 13:223–227.
Article13. Lekic PC, Kenny DJ, Barrett EJ. The influence of storage conditions on the clonogenic capacity of periodontal ligament cells: implications for tooth replantation. Int Endod J. 1998. 31:137–140.
Article14. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983. 65:55–63.
Article15. Andreasen JO. Analysis of pathogenesis and topography of replacement root resorption (ankylosis) after replantation of mature permanent incisors in monkeys. Swed Dent J. 1980a. 4:231–240.16. Andreasen JO. Analysis of topography of surface- and inflammatory root resorption after replantation of mature permanent incisors in monkeys. Swed Dent J. 1980b. 4:135–144.17. Birkedal-Hansen H. External root resorption caused by luxation of rat molars. Scand J Dent Res. 1973. 81:47–61.
Article18. Bornstein P. The cross-linking of collagen and elastin and its inhibition in osteolathyrism. Is there a relation to the aging process? Am J Med. 1970. 49:429–435.
Article19. Barrington EP, Meyer J. Recovery of the rat dental organ from experimental lathyrism. J Periodontol. 1966. 37:453–467.
Article20. Cho MI, Garant PR. The effect of beta-aminoproprionitrile on the periodontal ligament. I. Ultrastructure of fibroblasts and matrix. J Periodontal Res. 1984a. 19:247–260.
Article21. Cho MI, Garant PR. The effect of beta-aminoproprionitrile on the periodontal ligament: II. Radioautographic study of collagen secretion from fibroblasts. Anat Rec. 1984b. 209:41–52.
Article22. Ashkenazi M, Marouni M, Sarnat H. In vitro viability, mitogenicity and clonogenic capacity of periodontal ligament cells after storage in four media at room temperature. Endod Dent Traumatol. 2000. 16:63–70.
Article23. Hiltz J, Trope M. Vitality of human lip fibroblasts in milk, Hanks balanced salt solution and Viaspan storage media. Endod Dent Traumatol. 1991. 7:69–72.
Article24. Fischer S, Maclean AA, Liu M, Cardella JA, Slutsky AS, Suga M, Moreira JF, Keshavjee S. Dynamic changes in apoptotic and necrotic cell death correlate with severity of ischemia-reperfusion injury in lung transplantation. Am J Respir Crit Care Med. 2000. 162:1932–1939.
Article25. Hyon SH, Kim DH. Long-term preservation of rat pancreatic islets under physiological conditions. J Biotechnol. 2001. 85:241–246.
Article26. Sitasawad SL, Patole MS. Effect of cryopreservation on lipid peroxidation in chick cornea. Indian J Exp Biol. 1998. 36:1025–1027.27. Santanam N, Ramachandran S, Parthasarathy S. Oxygen radicals, antioxidants, and lipid peroxidation. Semin Reprod Endocrinol. 1998. 16:275–280.
Article28. Buttke TM, Trope M. Effect of catalase supplementation in storage media for avulsed teeth. Dent Traumatol. 2003. 19:103–108.
Article29. Chung WG. In vitro assessment on viability of human periodontal ligament cells after storage in chlorophyllin-added medium. 2002. Seoul: Yonsei Universtiy;Doctorial thesis.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The efficacy of programmed cryo-preservation under pressure in rat periodontal ligament cells
- Evaluation of the viability of periodontal ligament cell in rat teeth using slow cryopreservation method with magnetic field
- Evaluation of the Viability of Rat Periodontal Ligament Cells after Storing at 0℃/2 MPa Condition up to One Week: In Vivo MTT Method
- Evaluation of periodontal ligament cell viability in rat teeth after frozen preservation using in-vivo MTT assay
- Evaluation of periodontal ligament cell viability in rat teeth according to various extra-oral dry storage times using MTT assay