J Korean Acad Conserv Dent.
2004 May;29(3):239-248. 10.5395/JKACD.2004.29.3.239.
Local application of NK1 receptor antagonists and pulpal blood flow in cat
- Affiliations
-
- 1Department of Conservative Dentistry, School of Dentistry, Kyungpook National University, Korea. skykim@knu.ac.kr
- 2Department of Oral Physiology, School of Dentistry, Kyungpook National University, Korea.
- 3Department of Conservative Dentistry, The Institute of Oral Health Science, Samsung Medical Center, Sungkyunkwan University, School of Medicine, Korea.
- KMID: 1987100
- DOI: http://doi.org/10.5395/JKACD.2004.29.3.239
Abstract
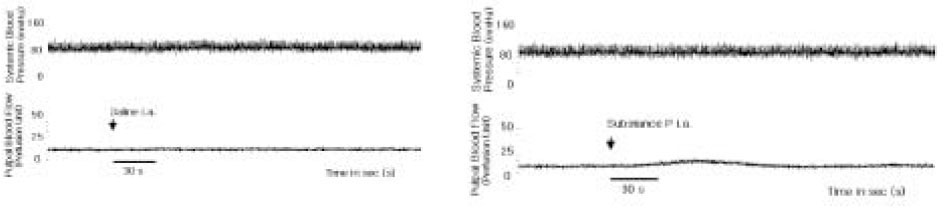
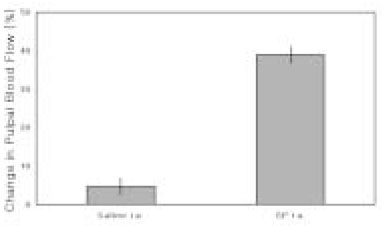
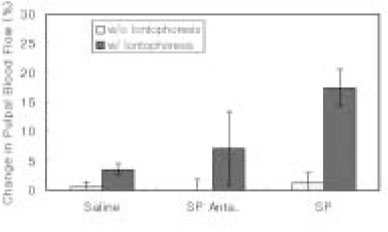
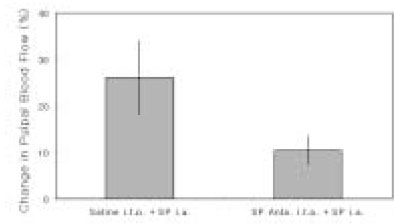
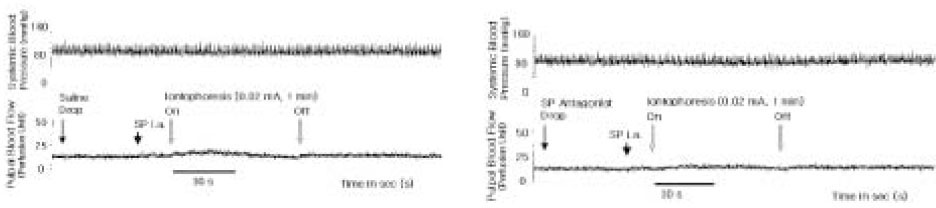
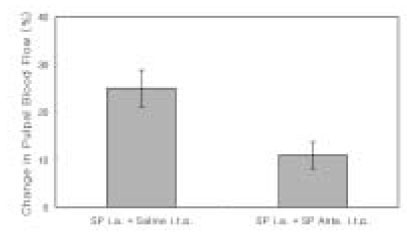
- The purpose of this study was to investigate the influence of NK1 receptor antagonists on the pulpal blood flow (PBF) when applied iontophoretically through the dentinal cavity of the teeth in order to understand whether iontophoretically applied NK1 receptor antagonists can control the pulpal inflammation. Eleven cats were anesthetized with alpha-chloralose and urethane, and substance P (SP) was administered to the dental pulp through the catheterized lingual artery in doses that caused PBF change without the influence of systemic blood pressure. NK1 receptor antagonists were applied iontophoretically to the prepared dentinal cavity of ipsilateral canine teeth of the drug administration, and PBF was monitored. Data were analyzed statistically with paired t-test. PBF increase after iontophoretic application of the NK1 receptor antagonists followed by the intra-arterial administration of SP was significantly less than PBF increase after iontophoretic application of the 0.9% saline followed by the intra-arterial administration of SP as a control (p < 0.05). Iontophoretic application of the NK1 receptor antagonists (0.2~3.4 mM) following the intra-arterial administration of SP resulted in less increase of PBF than the iontophoretic application of the 0.9% saline following the intra-arterial administration of SP as a control (p < 0.05). Therefore, the results of the present study provide evidences that the iontophoretic application is an effective method to deliver drugs to the dental pulp, and that iontophoretically applied NK1 receptor antagonists block SP-induced vasodilation effectively. The above results show the possibility that the iontophoretical application of NK1 receptor antagonists can control the neurogenic inflammation in the dental pulp.
Keyword
MeSH Terms
Figure
Reference
-
1. Heyeraas KJ, Kvinnsland I. Tissue pressure and blood flow in pulpal inflammation. Proc Finn Dent Soc. 1992. 88:Suppl 1. 393–401.2. Kim S. Neurovascular interactions in the dental pulp in health and inflammation. J Endod. 1990. 16(2):48–53.
Article3. Foreman JC, Jordan CC. Neurogenic inflammation. Trends Pharmacol Sci. 1984. 5:116–119.
Article4. V Euler US, Gaddum JH. An unidentified depressor substance in certain tissue extracts. J Physiol. 1931. 72:74–87.
Article5. Olgart L, Gazelius B, Brodin E, Nilsson G. Release of substance P-like immunoreactivity from the Dental Pulp. Acta Physiol Scand. 1977. 101:510–512.
Article6. Heyeraas KJ, Kim S, Raab WHM, Byers MR, Liu M. Effect of electrical tooth stimulation on blood flow, interstitial fluid pressure and substance P and CGRP-immunoreactive nerve fibers in the low compliant cat dental pulp. Microvasc Res. 1994. 47:329–343.
Article7. Lembeck F, Gamse R. Substance P in Peripheral Sensory Processes. Ciba Found Symp. 1982. (91):35–54.
Article8. Kim S, Dorscher-Kim JE, Liu MT, Trowbridge HO. Biphasic pulp blood-flow response to substance P in the dog as measured with a radiolabelled, microsphere injection method. Arch Oral Biol. 1988. 33:305–309.
Article9. Henry JL. Substance P and inflammatory pain: potential of substance P antagonists as analgesics. Agents Actions Suppl. 1993. 41:75–87.10. Kim SK, Karabucak B, Welsch H, Simchon S, Kim S. Intracellular mechanism of substance P-induced vasodilatation in bovine dental pulp. J Endod. 2001. 27:231.11. Berggreen E, Heyeraas KJ. The role of sensory neuropeptides and nitric oxide on pulpal blood flow and tissue pressure in the ferret. J Dent Res. 1999. 78(9):1535–1543.
Article12. Otsuka M, Yanagisawa M. Does substance P act as a pain transmitter? Trends Pharmacol Sci. 1987. 8:506–510.
Article13. Buck SH, Burcher E. The tachykinins: a family of peptides with a brood of 'receptors'. Trends Pharmacol Sci. 1986. 7:65–68.
Article14. Watling KJ, Krause JE. The rising sun shines on substance P and related peptides. Trends Pharmacol Sci. 1993. 14:81–84.
Article15. Hokfelt T, Pernow B, Wahren J. Substance P: a pioneer amongst neuropeptides. J Intern Med. 2001. 249:27–40.
Article16. Regoli D, Drapeau G, Dion S, Couture R. New selective agonist for neurokinin receptors: pharmacological tools for receptor characterization. Trends Pharmacol Sci. 1988. 9:290–295.
Article17. Kim SK, Ang L, Hsu YY, Kim S. Characterization of NK1 receptor antagonists on pulpal hemodynamics in cats. J Endod. 1995. 21(4):229.
Article18. Berggreen E, Heyeraas KJ. Effect of the sensory neuropeptide antagonists h-CGRP((8-37)) and SR 140.33 on pulpal and gingival blood flow in ferrets. Arch Oral Biol. 2000. 45:537–542.
Article19. Alia S, Azérad J, Pollin B. Effects of RPR 100893, a potent NK1 antagonist, on the jaw-opening reflex in the guinea pig. Brain Res. 1998. 787(1):99–106.
Article20. Wiesenfeld-Hallin Z, Villar MJ, Hokfelt T. The effects of intrathecal galanin and C-fiber stimulation on the flexor reflex in the rat. Brain Res. 1989. 486:205–213.
Article21. Wiesenfeld-Hallin Z, Xu XJ, Håkanson R, Feng DM, Forker T. The specific antagonistic effect of intrathecal Spantide II on substance P and C-fiber conditioning stimulation-induced facilitation on the nociceptive flexor reflex in the rat. Brain Res. 1990. 526:284–290.
Article22. Yonehara N, Sawada T, Matsuura H, Inoki R. Influence of electro-acupuncture on the release of substance P and the potential evoked by tooth pulp stimulation in the trigeminal nucleus caudalis of the rabbit. Neurosci Lett. 1992. 142(1):53–56.
Article23. Beattie DT, Connor HE, Hagan RM. Recent development in tachykinin NK1 receptor antagonists: prospects for the treatment of migraine headache. Can J Physiol Pharmacol. 1995. 73:871–877.
Article24. Kim S, Liu M, Simchon S, Dörscher-Kim JE. Effects of selected inflammatory mediators on blood flow and vascular permeability in the dental pulp. Proc Finn Dent Soc. 1992. 88:Suppl 1. 387–392.25. Olgart L, Edwall L, Gazelius B. Involvement of afferent nerves in pulpal blood-flow reactions in response to clinical and experimental procedures in the cat. Arch Oral Biol. 1991. 36:575–581.
Article26. Liu M, Pertl C, Markowitz K, Dörscher-Kim JE, Kim S. The effects of capsaicin on pulpal blood flow. Proc Finn Dent Soc. 1992. 88:Suppl 1. 463–467.27. Raab WH. Temperature related changes in pulpal microcirculation. Proc Finn Dent Soc. 1992. 88:Suppl 1. 469–479.28. Fazekas A, Gyorfi A, Irmes F, Rosivall L. Effect of substance P administration on vascular permeability in the rat dental pulp and submandibular gland. Proc Finn Dent Soc. 1992. 88:481–486.29. Vongsavan N, Matthews B. Changes in pulpal blood flow and in fluid flow through dentine produced by autonomic and sensory nerve stimulation in the cat. Proc Finn Dent Soc. 1992. 88:Suppl 1. 491–497.30. Lembeck F, Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979. 310:175–183.
Article31. Louis SM, Jamieson A, Russell NJW, Dockray GJ. The role of substance P and calcitonin gene-related peptide in neurogenic plasma extravasation and vasodilatation in the rat. Neuroscience. 1989. 32(3):581–586.
Article32. Olgart L, Gazelius B. Enhanced vasodilation in sensory denervated tooth pulp by substance P (SP). Reg Pep. 1988. 22:(Abstract) 137.33. Kim S. Regulation of pulpal blood flow. J Dent Res. 1985. 64 Spec No:590–596.
Article34. Kim S. Microcirculation of the dental pulp in health and disease. J Endod. 1985. 11:465–471.
Article35. Kim S, Dörscher-Kim JE. Hemodynamic regulation of the dental pulp in a low compliance environment. J Endod. 1989. 15(9):404–408.
Article36. Kim S, Trowbridge H, Dörscher-Kim JE. The effects of 5-hydroxytryptamine on pulpal hemodynamics in dogs. J Dent Res. 1986. 65:682–685.37. Kim S, Fan FC, Chen RYZ, Simchon S, Schuessler GB, Chien S. Symposium: 3. Effects of changes in systemic hemodynamic parameters in pulpal hemodynamics. J Endod. 1980. 6:394–399.
Article38. Kim S. Regulation of blood flow of the dental pulp: macrocirculation and microcirculation studies [Ph. D. dissertation]. 1981. New York, NY: Columbia University.39. Håkanson R, Leander S, Andersson RG, Hörig J. Substance P antagonists release histamine from peritoneal mast cells. Acta Physiol Scand. 1983. 117:319–320.
Article40. Kostouros GD. Iontophoretic delivery of pharmacological agents to the dental pulp, an experimental study in the rat. 1996. Stockholm, Sweden: Department of Physiology and Pharmacology, Karolinska Institutet;1–39.41. Vongsavan N, Matthews B. The permeability of cat dentine in vivo and in vitro. Arch Oral Biol. 1991. 36:641–646.
Article42. Lindblad LE, Ekenvall L, Ancker K, Rohman H, Oberg PA. Laser Doppler flow-meter assessment of iontophoretically applied norepinephrine on human finger skin circulation. J Invest Dermatol. 1986. 87:634–636.
Article43. Walmsley D, Wiles PG. Early loss of neurogenic inflammation in the human diabetic foot. Clin Sci (Lond). 1991. 80:605–610.
Article44. Parkhouse N, Lequesne PM. Quantitative objective assessment of peripheral nociceptive C fibre function. J Neurol Neurosurg Psychiatry. 1988. 51:28–34.
Article45. Westerman RA, Widdop RE, Hogan C, Zimmet P. Non-invasive tests of neurovascular function: reduced responses in diabetes mellitus. Neurosci Lett. 1987. 81:177–182.
Article46. Roberts RG, Westerman RA, Widdop RE, Kotzmann RR, Payne R. Effects of capsaicin on cutaneous vasodilator responses in humans. Agents Actions. 1992. 37:53–59.
Article47. Westerman RA, Widdop RE, Hannaford J, Low A, Roberts RG, Kent P, Sideris K, Yip T, Hales JR, Stephens FR. Laser Doppler velocimetry in the measurement of neurovascular function. Australas Phys Eng Sci Med. 1988. 11:53–66.48. Lembeck F, Folkers K, Donnerer J. Analgesic effect of antagonists of substance P. Biochem Biophys Res Commun. 1981. 103:1318–1321.
Article49. Lembeck F, Donnerer J, Bartho L. Inhibition of neurogenic vasodilation and plasma extravasation by substance P antagonists, somatostatin and [D-Met2, Pro5] encephalinamide. Eur J Pharmacol. 1982. 85:171–176.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The influence of epinephrine concentration in local anesthetics on pulpal and gingival blood flows
- Maturation of the Neurokinin 1 (NK1) Receptor Immunoreactive Amacrine Cells in the Rat Retina during Postnatal Development
- Effect of local anesthesia on pulpal blood flow in mechanically stimulated teeth
- Anti-nociceptive effects of dual neuropeptide antagonist therapy in mouse model of neuropathic and inflammatory pain
- Management of Chemotherapy Induced Nausea and Vomiting