J Korean Acad Conserv Dent.
2009 Nov;34(6):491-499. 10.5395/JKACD.2009.34.6.491.
Comparison of viability of oral epithelial cells stored by different freezing methods
- Affiliations
-
- 1Department of Conservative Dentistry, College of Dentistry, Yonsei University, Korea. andyendo@yuhs.ac
- KMID: 1986612
- DOI: http://doi.org/10.5395/JKACD.2009.34.6.491
Abstract
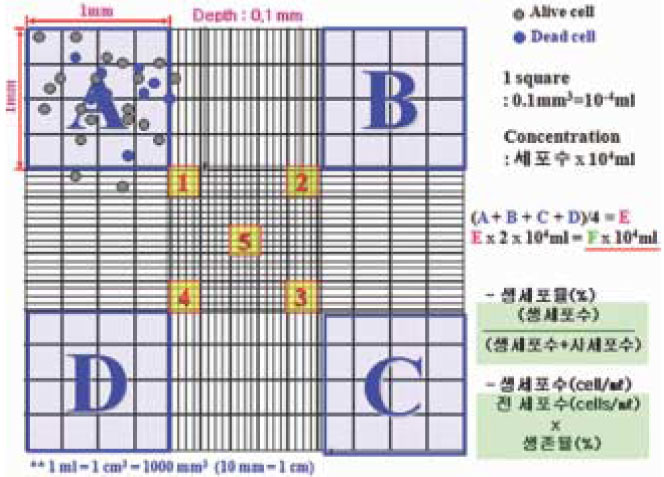
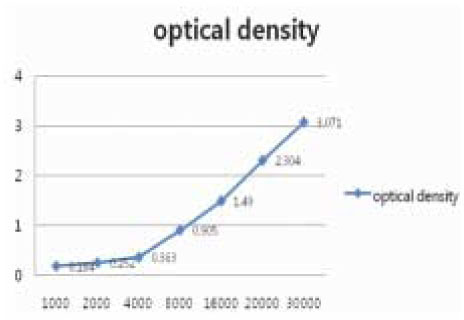
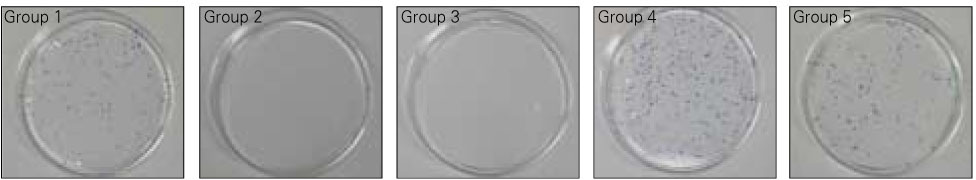
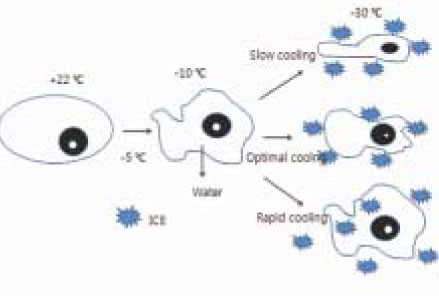
- This study examined the influence of the storage methods on the viability of oral epithelial cells using conventional cell freezing storage, slow freezing preservation, rapid freezing preservation, and slow freezing preservation with a pressure of 2 Mpa or 3 Mpa. The cell viability was evaluated by cell counting, WST-1 and the clonogenic capacity after 6 days of freezing storage. After 6 days, the frozen cells were thawed rapidly, and the cell counting, WST-1, and clonogenic capacity values were measured and compared. 1. The results from cell counting demonstrated that conventional cryopreservation, slow freezing under a 2 Mpa pressure and slow freezing under a 3 Mpa pressure showed significantly higher values than slow freezing preservation and rapid freezing preservation (p<0.05). 2. The results from the optical density by WST-1 demonstrated that slow freezing under a 2 Mpa pressure showed significantly higher values than slow freezing preservation and rapid freezing preservation (p<0.05). 3. The clonogenic capacity demonstrated that slow freezing under a 2 Mpa pressure showed significantly higher values than slow freezing preservation and rapid freezing preservation (p<0.05).
Keyword
Figure
Cited by 1 articles
-
The evaluation of periodontal ligament cells of rat teeth after low-temperature preservation under high pressure
Jin-Ho Chung, Jin Kim, Seong-Ho Choi, Eui-Seong Kim, Jiyong Park, Seung-Jong Lee
J Korean Acad Conserv Dent. 2010;35(4):285-294. doi: 10.5395/JKACD.2010.35.4.285.
Reference
-
1. Kim JW, Kim US, Kim J, Lee SJ. Evaluation of Periodontal Ligament Cell Viability in Rat Teeth after Frozen Preservation using in-vivo MTT Assay. J Korean Acad Conserv Dent. 2006. 31:192–202.
Article2. Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol. 1984. 247:C125–C142.
Article3. Farrant J. Water transport and cell survival in cryobiological procedures. Philos Trans R Soc Lond B Biol Sci. 1977. 278(959):191–205.
Article4. Fujikawa S. "Freeze-fracture and etching studies on membrane damage on human erythrocytes caused by formation of intracellular ice.". Cryobiology. 1980. 17(4):351–362.
Article5. Diller KR, Cravalho EG. A cryomicroscope for the study of freezing and thawing processes in biological cells. Cryobiology. 1970. 7(4):191–199.
Article6. Kawata T. Tooth transplantation by teeth bank-approach to human-Hiroshima. 2005. Department of Orthodontics, Hiroshima University School of Dentistry.7. Ahn HJ, Kim US, Kim J, Kim DW, Kim KY, Lee CY, Lee SJ. Evaluation of the Viability of Periodontal Ligament Cell in Rat Teeth using Slow Cryopreservation Method with Magnetic Field. J Korean Acad Conserv Dent. 2008. 33:332–340.
Article8. Pribenszky C, Molnar M, Cseh S, Solti L. Improving post-thaw survival of cryopreserved mouse blastocysts by hydrostatic pressure challenge. Anim Reprod Sci. 2005. 87(1-2):143–150.
Article9. Pribenszky C, Molnar M, Ulrich P, et al. Pressure assisted cryopreservation. Reprod Domest Anim. 2005. 40:228–344.10. Kuo YH, Pribenszky Cs, Huang SY. Higher litter size is achieved by the insemination of high hydrostatic pressure-treated frozen-thawed boar semen. Theriogenology. 2008. 70:1395–1396.
Article11. Berridge MV, Tan AS, McCoy KD, Wang R. The biochemical and cellular basis of cell proliferation assays that use tetrazolium salts. Biochemica (Indianapolis). 1996. 4:15–20.12. Alotto D, Ariotti S, Graziano S, Verrua R, Stella M, Magliacani G, Castagnoli C. The role of quality control in a skin bank: tissue viability determination. Cell Tissue Bank. 2002. 3(1):3–10.13. Czochrowska EM, Stenvik A, et al. "Outcome of tooth transplantation: survival and success rates 17-41 years posttreatment.". Am J Orthod Dentofacial Orthop. 2002. 121(2):110–119. quiz 193.
Article14. Jonsson T, Sigurdsson TJ. "Autotransplantation of premolars to premolar sites. A long-term follow-up study of 40 consecutive patients.". Am J Orthod Dentofacial Orthop. 2004. 125(6):668–675.
Article15. Mazur P. "Equilibrium, quasi-equilibrium, and non-equilibrium freezing of mammalian embryos.". Cell Biophys. 1990. 17(1):53–92.
Article16. Mazur P, Leibo SP, Chu EH. A two-factor hypothesis of freezing injury. Evidence from Chinese hamster tissue-culture cells. Exp Cell Res. 1972. 71(2):345–355.17. Rapatz G, Sullivan JJ, et al. "Preservation of erythrocytes in blood containing various cryoprotective agents, frozen at various rates and brought to a given final temperature.". Cryobiology. 1968. 5(1):18–25.
Article18. Thorpe PE, Knight SC, et al. "Optimal conditions for the preservation of mouse lymph node cells in liquid nitrogen using cooling rate techniques.". Cryobiology. 1976. 13(2):126–133.
Article19. Leibo SP, McGrath JJ, et al. "Microscopic observation of intracellular ice formation in unfertilized mouse ova as a function of cooling rate.". Cryobiology. 1978. 15(3):257–271.
Article20. Abe F, Kato C, Horikoshi K. Pressure regulated metabolism in microorganisms. Trends Microbiol. 1999. 7(11):447–453.21. Aldridge BE, Bruner LJ. Pressure effects on mechanisms of charge transport across bilayer membranes. Biochem Biophys Acta. 1985. 817(2):343–354.
Article22. Huang SY, Pribenszky C, Kuo YH, et al. The effect of hydrostatic pressure treatment on the protein profile of boar spermatozoa before and after freezing. Theriogenology. 2008. 70:1391.
Article23. Parsell DA, Lindquist S. The function of heat shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Ann Rev Genet. 1993. 27:437–496.
Article24. Pelham HR. Heat shock proteins: coming in from cold. Nature. 1988. 332:776–777.25. Du Y, Lin L, Schmidt M, Bogh IB, Kragh PM, Sorensen CB, Li J, Purup S, Pribenszky C, Molnar M, Kuwayama M, Zhang X, Yang H, Bolund L, Vajta G. High hydrostatic pressure treatment of porcine oocytes before handmade cloning improves developmental competence and cryosurvival. Cloning Stem Cells. 2008. 10(3):325–330.
Article26. Lee YE, Kim US, Kim J, Han SH, Lee SJ. The Efficacy of Programmed Cryo-preservation under Pressure in Rat Periodontal Ligament Cells. J Korean Acad Conserv Dent. 2009. 34:356–363.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of temperature and medium on the viability of Mycobacterium leprae during long term-storage
- Freezing and Thawing Conditions of Rat Hepatocytes
- The effect of steroids on the viability of endothelial cells of stored cornea
- Evaluation of vitrification for cryopreservation of teeth
- Cellular Viability of Cryopreserved Porcine Valve According to Warm Ischemic Time