Korean J Gastroenterol.
2014 Oct;64(4):182-188. 10.4166/kjg.2014.64.4.182.
Necroptosis in Liver and Pancreatic Diseases
- Affiliations
-
- 1Department of Internal Medicine, Hallym University College of Medicine, Chuncheon, Korea.
- 2Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea. hschoi96@hanyang.ac.kr
- KMID: 1979202
- DOI: http://doi.org/10.4166/kjg.2014.64.4.182
Abstract
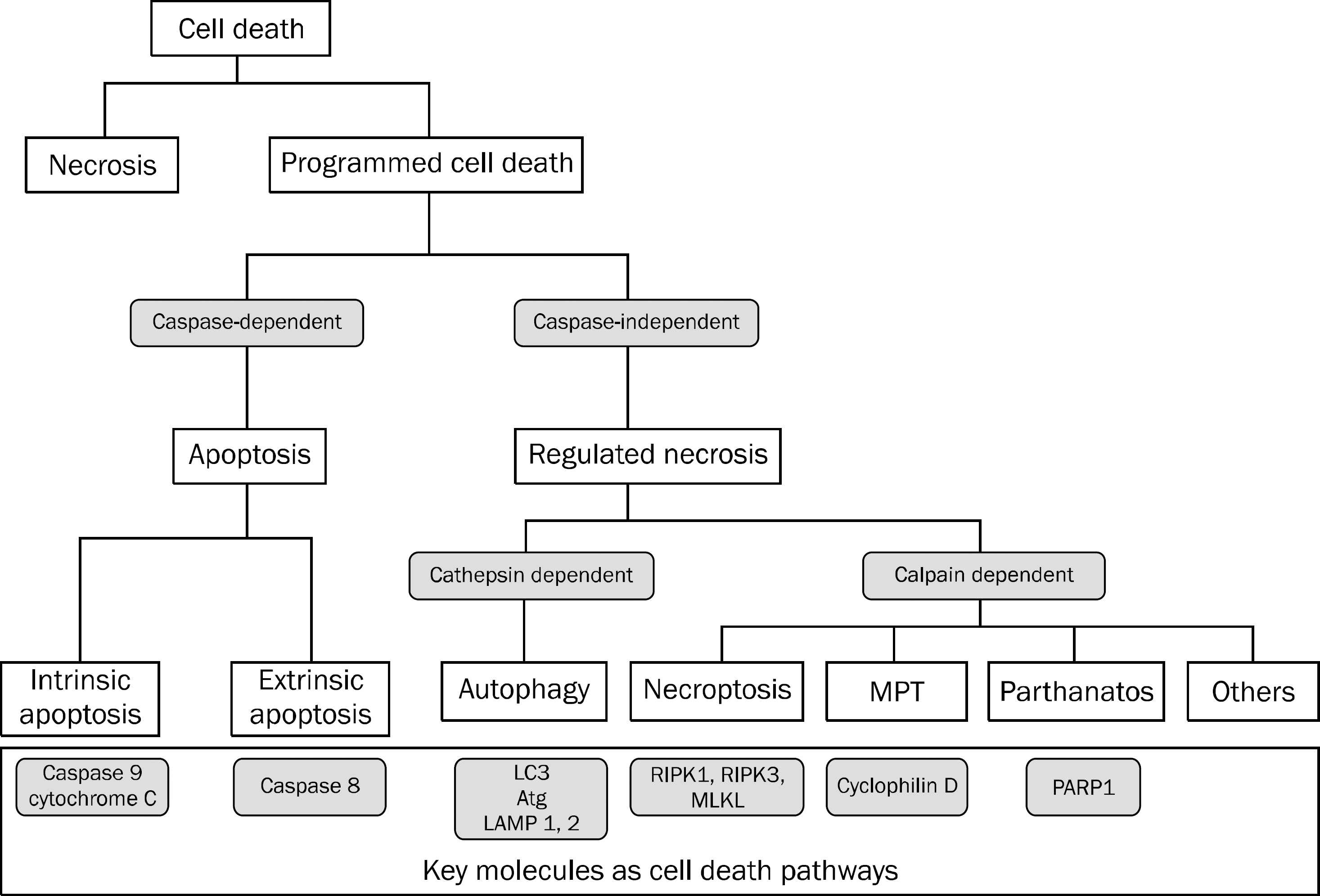
- Cell death is an integral part of life of an organism that is necessary to maintain organs and tissues. Apoptosis, autophagy, and necrosis were noted as three morphologically distinct types of cell death. Apoptosis is a well identified process that is driven by programmed molecular mechanism. Until now, the investigators believed that necrosis was not a programmed molecular event. However, recently, an alternative death pathway called 'necroptosis' was delineated and proposed as a form of 'programmed necrosis'. According to the recent recommendations by the Nomenclature Committee of Cell Death, this term denotes necrotic cell death dependent on receptor-interacting protein kinase (RIPK3). Its role in a variety of diseases, such as ischemia-perfusion injury, infection, inflammatory bowel disease, pancreatitis, steatohepatitis etc., is being elucidated. Necroptosis is currently attracting the attention of the scientific community. Herein we discuss the clinical implications and the role of necroptosis in gastrointestinal tract focusing on liver and pancreatic diseases.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Kerr JF. Shrinkage necrosis: a distinct mode of cellular death. J Pathol. 1971; 105:13–20.
Article2. Bortner CD, Cidlowski JA. Cellular mechanisms for the re-pression of apoptosis. Annu Rev Pharmacol Toxicol. 2002; 42:259–281.
Article3. Kitanaka C, Kuchino Y. Caspase-independent programmed cell death with necrotic morphology. Cell Death Differ. 1999; 6:508–515.
Article4. Zhang DW, Shao J, Lin J, et al. RIP3, an energy metabolism regu-lator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009; 325:332–336.
Article5. Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of non-apoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005; 1:112–119.
Article6. Galluzzi L, Kroemer G. Necroptosis: a specialized pathway of programmed necrosis. Cell. 2008; 135:1161–1163.
Article7. Galluzzi L, Vitale I, Abrams JM, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012; 19:107–120.
Article8. Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, Yellon DM. Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther. 2007; 21:227–233.
Article9. Linkermann A, Bräsen JH, Himmerkus N, et al. Rip1 (receptor-in-teracting protein kinase 1) mediates necroptosis and contrib-utes to renal ischemia/reperfusion injury. Kidney Int. 2012; 81:751–761.
Article10. Linkermann A, Bräsen JH, De Zen F, et al. Dichotomy between RIP1- and RIP3-mediated necroptosis in tumor necrosis factor-α- induced shock. Mol Med. 2012; 18:577–586.11. Wu J, Huang Z, Ren J, et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013; 23:994–1006.
Article12. Günther C, Martini E, Wittkopf N, et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature. 2011; 477:335–339.
Article13. von Montfort C, Matias N, Fernandez A, et al. Mitochondrial GSH determines the toxic or therapeutic potential of superoxide scav-enging in steatohepatitis. J Hepatol. 2012; 57:852–859.14. Smith CC, Yellon DM. Necroptosis, necrostatins and tissue injury. J Cell Mol Med. 2011; 15:1797–1806.
Article15. Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014; 370:455–465.
Article16. Vercammen D, Beyaert R, Denecker G, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998; 187:1477–1485.
Article17. Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013; 1833:3448–3459.
Article18. Han J, Zhong CQ, Zhang DW. Programmed necrosis: backup to and competitor with apoptosis in the immune system. Nat Immunol. 2011; 12:1143–1149.
Article19. Silke J, Strasser A. The FLIP side of life. Sci Signal. 2013; 6:pe2.
Article20. Vanlangenakker N, Vanden Berghe T, Bogaert P, et al. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 2011; 18:656–665.
Article21. Holler N, Zaru R, Micheau O, et al. Fas triggers an alternative, cas-pase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000; 1:489–495.
Article22. Schworer SA, Smirnova II, Kurbatova I, et al. Toll-like receptor-mediated down-regulation of the deubiquitinase cylin-dromatosis (CYLD) protects macrophages from necroptosis in wild-derived mice. J Biol Chem. 2014; 289:14422–14433.
Article23. Khan N, Lawlor KE, Murphy JM, Vince JE. More to life than death: molecular determinants of necroptotic and non-necroptotic RIP3 kinase signaling. Curr Opin Immunol. 2014; 26:76–89.
Article24. Gerlach B, Cordier SM, Schmukle AC, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011; 471:591–596.
Article25. Mevissen TE, Hospenthal MK, Geurink PP, et al. OTU deubiquiti-nases reveal mechanisms of linkage specificity and enable ubiquitin chain restriction analysis. Cell. 2013; 154:169–184.
Article26. Oberst A, Green DR. It cuts both ways: reconciling the dual roles of caspase 8 in cell death and survival. Nat Rev Mol Cell Biol. 2011; 12:757–763.
Article27. Oberst A, Dillon CP, Weinlich R, et al. Catalytic activity of the cas-pase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011; 471:363–367.
Article28. Zhao J, Jitkaew S, Cai Z, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012; 109:5322–5327.
Article29. Sun L, Wang H, Wang Z, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012; 148:213–227.
Article30. Dillon CP, Oberst A, Weinlich R, et al. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 2012; 1:401–407.
Article31. Welz PS, Wullaert A, Vlantis K, et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011; 477:330–334.
Article32. Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the re-lease of damage-associated molecular patterns and its physio-logical relevance. Immunity. 2013; 38:209–223.
Article33. Kaiser WJ, Upton JW, Long AB, et al. RIP3 mediates the embry-onic lethality of caspase-8-deficient mice. Nature. 2011; 471:368–372.
Article34. Kaiser WJ, Upton JW, Mocarski ES. Viral modulation of programmed necrosis. Curr Opin Virol. 2013; 3:296–306.
Article35. Thapa RJ, Nogusa S, Chen P, et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A. 2013; 110:E3109–E3118.
Article36. Li S, Zhang L, Yao Q, et al. Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature. 2013; 501:242–246.
Article37. He S, Wang L, Miao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009; 137:1100–1111.38. Shirinzadeh H, Eren B, Gurer-Orhan H, Suzen S, Ozden S. Novel indole-based analogs of melatonin: synthesis and in vitro anti-oxidant activity studies. Molecules. 2010; 15:2187–2202.
Article39. Kim HJ, Koo SY, Ahn BH, et al. NecroX as a novel class of mitochondrial reactive oxygen species and ONOO− scavenger. Arch Pharm Res. 2010; 33:1813–1823.40. Choi JM, Park KM, Kim SH, et al. Effect of necrosis modulator necrox-7 on hepatic ischemia-reperfusion injury in beagle dogs. Transplant Proc. 2010; 42:3414–3421.
Article41. Gukovsky I, Li N, Todoric J, Gukovskaya A, Karin M. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013; 144:1199–1209.e4.
Article42. Mareninova OA, Hermann K, French SW, et al. Impaired auto-phagic flux mediates acinar cell vacuole formation and trypsi-nogen activation in rodent models of acute pancreatitis. J Clin Invest. 2009; 119:3340–3355.
Article43. Moscat J, Diaz-Meco MT. p62: a versatile multitasker takes on cancer. Trends Biochem Sci. 2012; 37:230–236.
Article44. Farkas T, Daugaard M, Jäättelä M. Identification of small molecule inhibitors of phosphatidylinositol 3-kinase and autophagy. J Biol Chem. 2011; 286:38904–38912.
Article45. Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014; 147:765–783.e4.
Article46. Wang H, Sun L, Su L, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phos-phorylation by RIP3. Mol Cell. 2014; 54:133–146.
Article47. Luedde M, Lutz M, Carter N, et al. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after my-ocardial infarction. Cardiovasc Res. 2014; 103:206–216.
Article48. Vucur M, Reisinger F, Gautheron J, et al. RIP3 inhibits inflammatory hepatocarcinogenesis but promotes cholestasis by con-trolling caspase-8- and JNK-dependent compensatory cell proliferation. Cell Rep. 2013; 4:776–790.
Article49. Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H. Receptor interacting protein kinase 3 is a critical early media-tor of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013; 58:2099–2108.
Article50. Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE. Absence of receptor interacting protein kinase 3 prevents etha-nol-induced liver injury. Hepatology. 2013; 57:1773–1783.
Article51. Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012; 82:1001–1007.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cell Death and Liver Disease
- Lyso-globotriaosylsphingosine induces endothelial dysfunction via autophagy-dependent regulation of necroptosis
- Programmed cell death in alcohol-associated liver disease
- Public Fear of Pancreatic Diseases: Causes and Clinical Outcomes at a Single Korean Center
- Ultrasonography of pancreatic disease